
What is the mass of one mole of barium acetate, \[Ba{\left( {{C_2}{H_3}{O_2}} \right)_2}\]?
Answer
521.4k+ views
Hint: We need to remember that the barium acetate is a chemical compound having the formula, \[Ba{\left( {{C_2}{H_3}{O_2}} \right)_2}\]. It is a salt of barium and acetic acid. And the barium acetate is mainly used for manufacturing and it is used for the preparation of other acetates.
Complete answer:
We also need to know that a mole is used to specify the amount of material and it contains \[6.022 \times {10^{23}}\] particles. Hence, one mole is equal to \[6.022 \times {10^{23}}\] particles and this number is called Avogadro number. The mole is mainly used to express the number of products and the reactants. Hence, it is a count of particles. The mass of one mole of substance is equal to the molar mass of the compound. Therefore, the number of moles is equal to the amount of samples.
As we know that the barium acetate is a chemical compound having the formula, \[Ba{\left( {{C_2}{H_3}{O_2}} \right)_2}\]. It is a salt of barium and acetic acid. And the barium acetate is mainly used for manufacturing and it is used for the preparation of other acetates. The mass of one mole of barium acetate is equal to its molecular mass.
The molar mass of barium, Ba \[ = 137\]
The molar mass of carbon, C\[ = 12\]
The molar mass of hydrogen, H\[ = 1\]
The molar mass of oxygen, O\[ = 16\]
To find the mass of barium sulphate, add the molar mass of elements. Therefore,
The mass of one mole of barium acetate \[ = 137 + 4 \times 12 + 6 + 4 \times 16\]
\[ = 255\]
Hence, the mass of one mole barium acetate is equal to \[255\]. Barium acetate is a strong electrolyte and it does not undergo hydrolysis. And the structure of barium acetate can be drawn as,
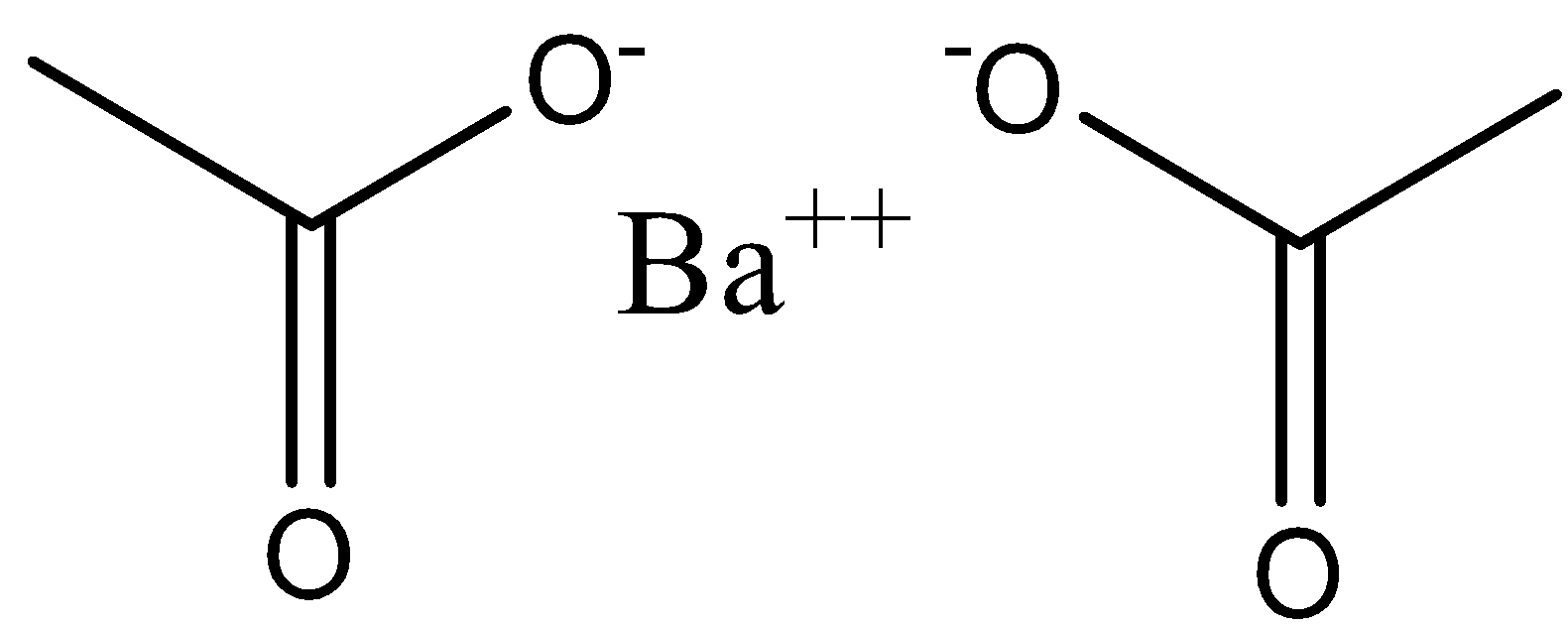
Note:
As we know that the mass of one mole of barium acetate is equal to its molecular mass. Hence, the mass of one mole of barium acetate is equal to \[255\]. The barium sulphate is used as a catalyst in organic synthesis. And the barium acetate is prepared by the reaction of acetic acid with barium carbonate. And the reaction can be written as,
\[BaC{O_3} + 2C{H_3}COOH \to {\left( {C{H_3}COO} \right)_2}Ba + C{O_2} + {H_2}O\]
Complete answer:
We also need to know that a mole is used to specify the amount of material and it contains \[6.022 \times {10^{23}}\] particles. Hence, one mole is equal to \[6.022 \times {10^{23}}\] particles and this number is called Avogadro number. The mole is mainly used to express the number of products and the reactants. Hence, it is a count of particles. The mass of one mole of substance is equal to the molar mass of the compound. Therefore, the number of moles is equal to the amount of samples.
As we know that the barium acetate is a chemical compound having the formula, \[Ba{\left( {{C_2}{H_3}{O_2}} \right)_2}\]. It is a salt of barium and acetic acid. And the barium acetate is mainly used for manufacturing and it is used for the preparation of other acetates. The mass of one mole of barium acetate is equal to its molecular mass.
The molar mass of barium, Ba \[ = 137\]
The molar mass of carbon, C\[ = 12\]
The molar mass of hydrogen, H\[ = 1\]
The molar mass of oxygen, O\[ = 16\]
To find the mass of barium sulphate, add the molar mass of elements. Therefore,
The mass of one mole of barium acetate \[ = 137 + 4 \times 12 + 6 + 4 \times 16\]
\[ = 255\]
Hence, the mass of one mole barium acetate is equal to \[255\]. Barium acetate is a strong electrolyte and it does not undergo hydrolysis. And the structure of barium acetate can be drawn as,
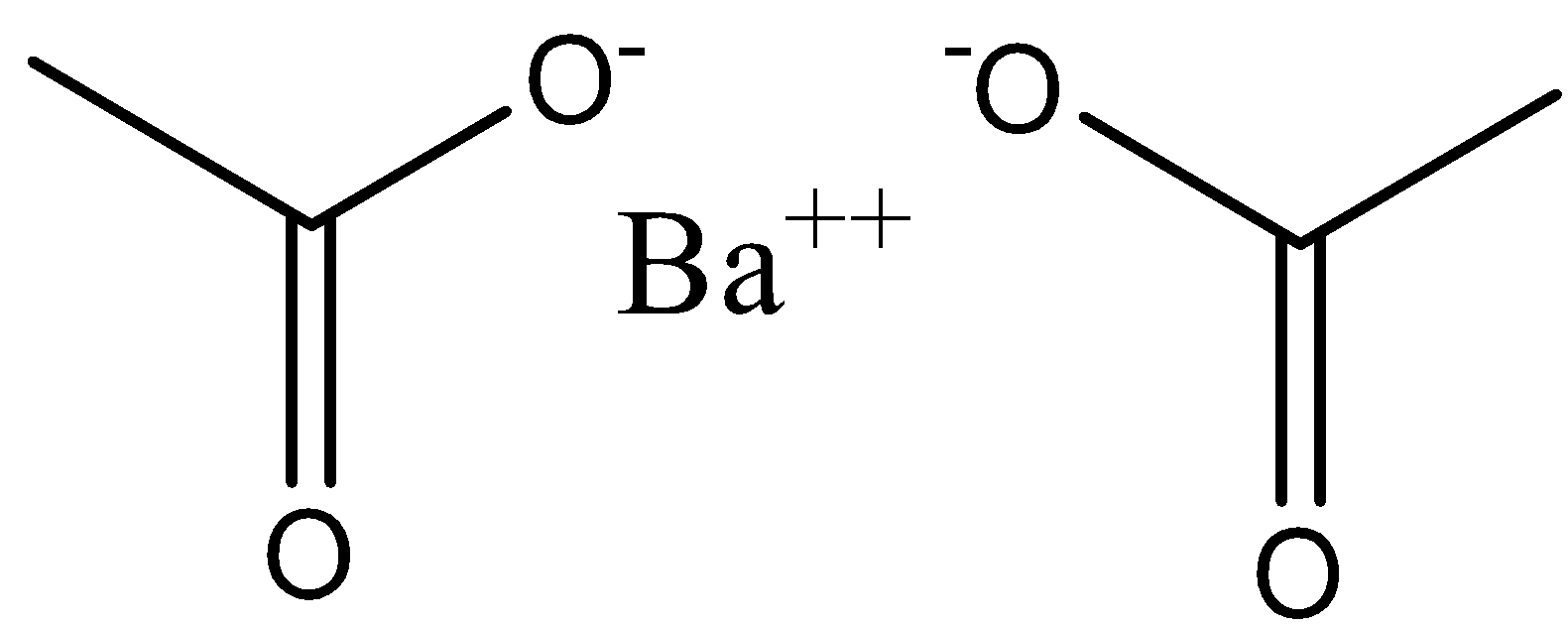
Note:
As we know that the mass of one mole of barium acetate is equal to its molecular mass. Hence, the mass of one mole of barium acetate is equal to \[255\]. The barium sulphate is used as a catalyst in organic synthesis. And the barium acetate is prepared by the reaction of acetic acid with barium carbonate. And the reaction can be written as,
\[BaC{O_3} + 2C{H_3}COOH \to {\left( {C{H_3}COO} \right)_2}Ba + C{O_2} + {H_2}O\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




