
What mass of Ag (atomic mass = 108) could be plated on a spoon from electrolysis of $AgN{{O}_{3}}$ solution by ampere current for 10 minutes.
(A)- $0.6715g$
(B)- $1.6715g$
(C)- $0.15g$
(D)- $0.815g$
Answer
573.6k+ views
Hint: The time for which the current is passed through the cell produces electrons within the silver nitrate solution, which further leads to the reduction of silver ions in the solution which is directly related to the moles of the metal produced.
Complete step by step answer:
It is given that the silver is deposited or coated on the spoon by electrolysis of silver nitrate solution. This is known as the electroplating process.
- This electrolysis process involves an electrolytic cell where the silver metal is anode and the spoon to be coated is the cathode. The silver nitrate solution is the electrolyte which allows the flow of ions, in which both the electrodes are dipped.
- Then, on passing current through the cell, the silver metal at anode loses its electrons and enters the silver nitrate solution, whereas the electrode rich in electrons, that is, the cathode gives its electrons to the $A{{g}^{+}}$ ions in the solution leading to the deposition of the metal at the cathode.
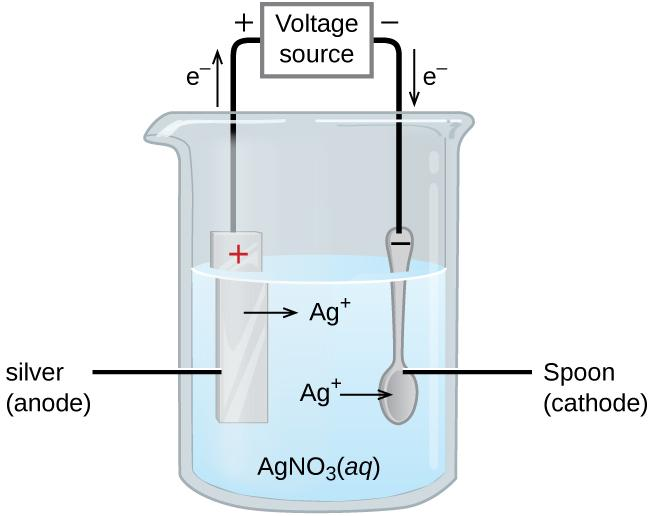
At anode, where the oxidation process occurs: $Ag(s)\to A{{g}^{+}}(aq)+{{e}^{-}}$
At cathode, where the reduction process occurs: $A{{g}^{+}}(aq)+{{e}^{-}}\to Ag(s)$
Therefore, this electrolytic reaction causes electroplating or thin coating of the metal on the conducting surface of the spoon.
- Here, the amount of current passed in the cell is related to the number of moles of electrons produced. Then, as the current is the rate of flow of charge. We get,
\[Q=I\times t=n\times F\]
where, $Q$ is the total charge (in coulombs), $I$ is current (in amperes), $t$ is time in seconds, $n$ is the number of moles of electrons, and $F$ is the Faraday constant.
So, given current that is passed for 10 minutes, the total charge is $(1A\times 10\times 60)=600\,C$.
In the reduction process, one mole of electrons generates one mole of silver on cathode. That is, 96500 C of electricity produces one mole of silver.
Then, 600 C of electricity will produce $=\dfrac{600}{96500}=0.006217\,\text{moles}$
So, mass of silver $\text{= moles }\times \text{ molar mass}\,\text{=}\,\text{0}\text{.00621}\times \text{108}\,\text{=}\,\text{0}\text{.6715}\,\text{g}$
Therefore, option (A)- 0.6715 g of silver is coated on the spoon from the electrolysis of silver nitrate solution for 10 minutes.
Note: This electroplating process is made use of in order to make the surface corrosion resistant, for strengthening, for attractive finish and purification of metal.
Complete step by step answer:
It is given that the silver is deposited or coated on the spoon by electrolysis of silver nitrate solution. This is known as the electroplating process.
- This electrolysis process involves an electrolytic cell where the silver metal is anode and the spoon to be coated is the cathode. The silver nitrate solution is the electrolyte which allows the flow of ions, in which both the electrodes are dipped.
- Then, on passing current through the cell, the silver metal at anode loses its electrons and enters the silver nitrate solution, whereas the electrode rich in electrons, that is, the cathode gives its electrons to the $A{{g}^{+}}$ ions in the solution leading to the deposition of the metal at the cathode.

At anode, where the oxidation process occurs: $Ag(s)\to A{{g}^{+}}(aq)+{{e}^{-}}$
At cathode, where the reduction process occurs: $A{{g}^{+}}(aq)+{{e}^{-}}\to Ag(s)$
Therefore, this electrolytic reaction causes electroplating or thin coating of the metal on the conducting surface of the spoon.
- Here, the amount of current passed in the cell is related to the number of moles of electrons produced. Then, as the current is the rate of flow of charge. We get,
\[Q=I\times t=n\times F\]
where, $Q$ is the total charge (in coulombs), $I$ is current (in amperes), $t$ is time in seconds, $n$ is the number of moles of electrons, and $F$ is the Faraday constant.
So, given current that is passed for 10 minutes, the total charge is $(1A\times 10\times 60)=600\,C$.
In the reduction process, one mole of electrons generates one mole of silver on cathode. That is, 96500 C of electricity produces one mole of silver.
Then, 600 C of electricity will produce $=\dfrac{600}{96500}=0.006217\,\text{moles}$
So, mass of silver $\text{= moles }\times \text{ molar mass}\,\text{=}\,\text{0}\text{.00621}\times \text{108}\,\text{=}\,\text{0}\text{.6715}\,\text{g}$
Therefore, option (A)- 0.6715 g of silver is coated on the spoon from the electrolysis of silver nitrate solution for 10 minutes.
Note: This electroplating process is made use of in order to make the surface corrosion resistant, for strengthening, for attractive finish and purification of metal.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




