
Marshall’s acid is:
(A) ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$
(B) ${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{7}}}$
(C) ${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{3}}}$
(D) ${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_8}$
Answer
583.8k+ views
Hint: Marshall’s acid is also known as peroxydisulfuric acid. Peroxy indicates that the ${\text{O}} - {\text{O}}$ linkage is present.
Step by step answer: ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ is known as sulphuric acid. Sulphuric acid is also known as hydrogen sulphate or oil of vitriol.
Thus, ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ is not Marshall’s acid.
Thus, option (A) is not correct.
${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{7}}}$ is known as disulphuric acid. Disulphuric acid is also known as pyrosulphuric acid.
Thus, ${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{7}}}$ is not Marshall’s acid.
Thus, option (B) is not correct.
${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{3}}}$ is known as thiosulphuric acid. Thiosulphuric acid is also known as hydroxidioxidosulfido sulphate.
Thus, ${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{3}}}$ is not Marshall’s acid.
Thus, option (C) is not correct.
${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_8}$ is known as peroxydisulfuric acid. Peroxydisulfuric acid is also known as Marshall’s acid.
Thus, ${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_8}$ is Marshall’s acid.
Thus, the correct option is (D) ${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_8}$.
Additional Information:
-Marshall’s acid is an inorganic compound. It is solid and colourless in appearance. It is a powerful oxidising agent. The salts of Marshall’s acid are industrially important.
-Marshall’s acid is named after Professor Hugh Marshall who invented it.
-Ways of production of Marshall’s acid are as follows:
-From potassium sulphate or ammonium sulphate in acidic solution.
-Oxidation of oleum with hydrogen peroxide or ozone.
-Electrolysing aqueous solution of sulphate ions.
-Reaction of chlorosulfonic acid with hydrogen peroxide.
-Electrolysis of sulphuric acid with platinum electrodes at high voltage.
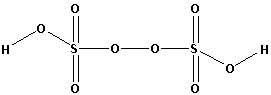
Note: Marshall’s acid in structural terms is written as ${\text{H}}{{\text{O}}_{\text{3}}}{\text{SOOS}}{{\text{O}}_{\text{3}}}{\text{H}}$. The oxidation state of sulphur is ${\text{ + 6}}$.
The structure of Marshall’s acid is as follows:
Peroxy indicates that the ${\text{O}} - {\text{O}}$ linkage is present.
Step by step answer: ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ is known as sulphuric acid. Sulphuric acid is also known as hydrogen sulphate or oil of vitriol.
Thus, ${{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}$ is not Marshall’s acid.
Thus, option (A) is not correct.
${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{7}}}$ is known as disulphuric acid. Disulphuric acid is also known as pyrosulphuric acid.
Thus, ${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{7}}}$ is not Marshall’s acid.
Thus, option (B) is not correct.
${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{3}}}$ is known as thiosulphuric acid. Thiosulphuric acid is also known as hydroxidioxidosulfido sulphate.
Thus, ${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_{\text{3}}}$ is not Marshall’s acid.
Thus, option (C) is not correct.
${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_8}$ is known as peroxydisulfuric acid. Peroxydisulfuric acid is also known as Marshall’s acid.
Thus, ${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_8}$ is Marshall’s acid.
Thus, the correct option is (D) ${{\text{H}}_{\text{2}}}{{\text{S}}_2}{{\text{O}}_8}$.
Additional Information:
-Marshall’s acid is an inorganic compound. It is solid and colourless in appearance. It is a powerful oxidising agent. The salts of Marshall’s acid are industrially important.
-Marshall’s acid is named after Professor Hugh Marshall who invented it.
-Ways of production of Marshall’s acid are as follows:
-From potassium sulphate or ammonium sulphate in acidic solution.
-Oxidation of oleum with hydrogen peroxide or ozone.
-Electrolysing aqueous solution of sulphate ions.
-Reaction of chlorosulfonic acid with hydrogen peroxide.
-Electrolysis of sulphuric acid with platinum electrodes at high voltage.
Note: Marshall’s acid in structural terms is written as ${\text{H}}{{\text{O}}_{\text{3}}}{\text{SOOS}}{{\text{O}}_{\text{3}}}{\text{H}}$. The oxidation state of sulphur is ${\text{ + 6}}$.
The structure of Marshall’s acid is as follows:
Peroxy indicates that the ${\text{O}} - {\text{O}}$ linkage is present.

Recently Updated Pages
Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Computer Science: Engaging Questions & Answers for Success

Class 10 Question and Answer - Your Ultimate Solutions Guide

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Trending doubts
A boat goes 24 km upstream and 28 km downstream in class 10 maths CBSE

State and explain Ohms law class 10 physics CBSE

Write a letter to the editor of a newspaper explaining class 10 english CBSE

Distinguish between soap and detergent class 10 chemistry CBSE

a Why did Mendel choose pea plants for his experiments class 10 biology CBSE

What is a "free hit" awarded for in limited-overs cricket?




