
Markovnikov’s rule for the addition of hydrogen halides to alkene states that the incoming hydrogen bonds to the:
A. \[{\text{s}}{{\text{p}}^{\text{2}}}\] carbon with the most hydrogens already
B. \[{\text{s}}{{\text{p}}^{\text{2}}}\] carbon with the fewest hydrogens already
C. \[{\text{s}}{{\text{p}}^3}\] carbon with the most hydrogens already
D. \[{\text{s}}{{\text{p}}^3}\] carbon with the few hydrogens already
Answer
569.4k+ views
Hint: Markovnikov’s rule for the addition of hydrogen halide to an asymmetric alkene. The addition of reagent takes place across the double bond. Markovnikov’s addition of hydrogen gives rise to a more stable carbocation.
Complete answer:
The addition of an asymmetric reagent to an asymmetric alkene takes place using Markovnikov’s rule.
Markovnikov’s rule states that when hydrogen halide is added to asymmetric alkene the acidic hydrogen gets added to less substituted carbon or carbon having more number of hydrogen atoms. While negative, the reagent gets added to the more substituted carbon atom of \[{\text{C = C}}\] the double bond. In the case of hydrogen halides, the negative part of the reagent is a halide. A more substituted carbon atom of \[{\text{C = C}}\] the double bond is the carbon which has less number of hydrogen atoms.
Consider the general reaction,
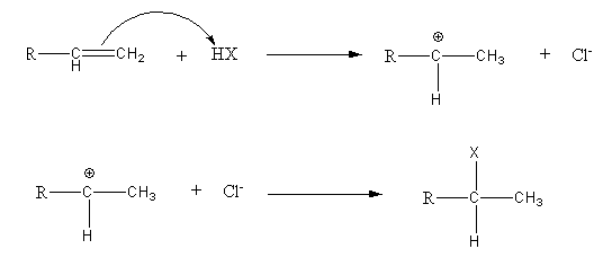
Thus, Markovnikov’s rule for the addition of hydrogen halides to alkene states that the incoming hydrogen bonds to the \[{\text{s}}{{\text{p}}^{\text{2}}}\] carbon with the most hydrogens already.
Thus, the correct option is (A) \[{\text{s}}{{\text{p}}^{\text{2}}}\] carbon with the most hydrogens already.
Note:
The first step of Markovnikov’s addition is the protonation of \[{\text{s}}{{\text{p}}^{\text{2}}}\] carbon with the most hydrogen already. This addition gives rise to more stable carbocation while protonation of \[{\text{s}}{{\text{p}}^{\text{2}}}\] carbon with the least hydrogens already gives rise to the least stable carbocation that is not favourable.
Complete answer:
The addition of an asymmetric reagent to an asymmetric alkene takes place using Markovnikov’s rule.
Markovnikov’s rule states that when hydrogen halide is added to asymmetric alkene the acidic hydrogen gets added to less substituted carbon or carbon having more number of hydrogen atoms. While negative, the reagent gets added to the more substituted carbon atom of \[{\text{C = C}}\] the double bond. In the case of hydrogen halides, the negative part of the reagent is a halide. A more substituted carbon atom of \[{\text{C = C}}\] the double bond is the carbon which has less number of hydrogen atoms.
Consider the general reaction,

Thus, Markovnikov’s rule for the addition of hydrogen halides to alkene states that the incoming hydrogen bonds to the \[{\text{s}}{{\text{p}}^{\text{2}}}\] carbon with the most hydrogens already.
Thus, the correct option is (A) \[{\text{s}}{{\text{p}}^{\text{2}}}\] carbon with the most hydrogens already.
Note:
The first step of Markovnikov’s addition is the protonation of \[{\text{s}}{{\text{p}}^{\text{2}}}\] carbon with the most hydrogen already. This addition gives rise to more stable carbocation while protonation of \[{\text{s}}{{\text{p}}^{\text{2}}}\] carbon with the least hydrogens already gives rise to the least stable carbocation that is not favourable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




