
What is the major product of the reaction?
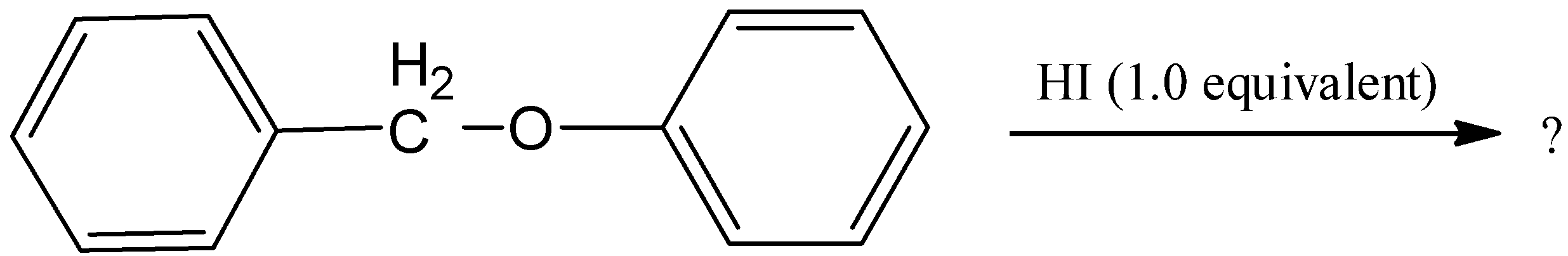
I
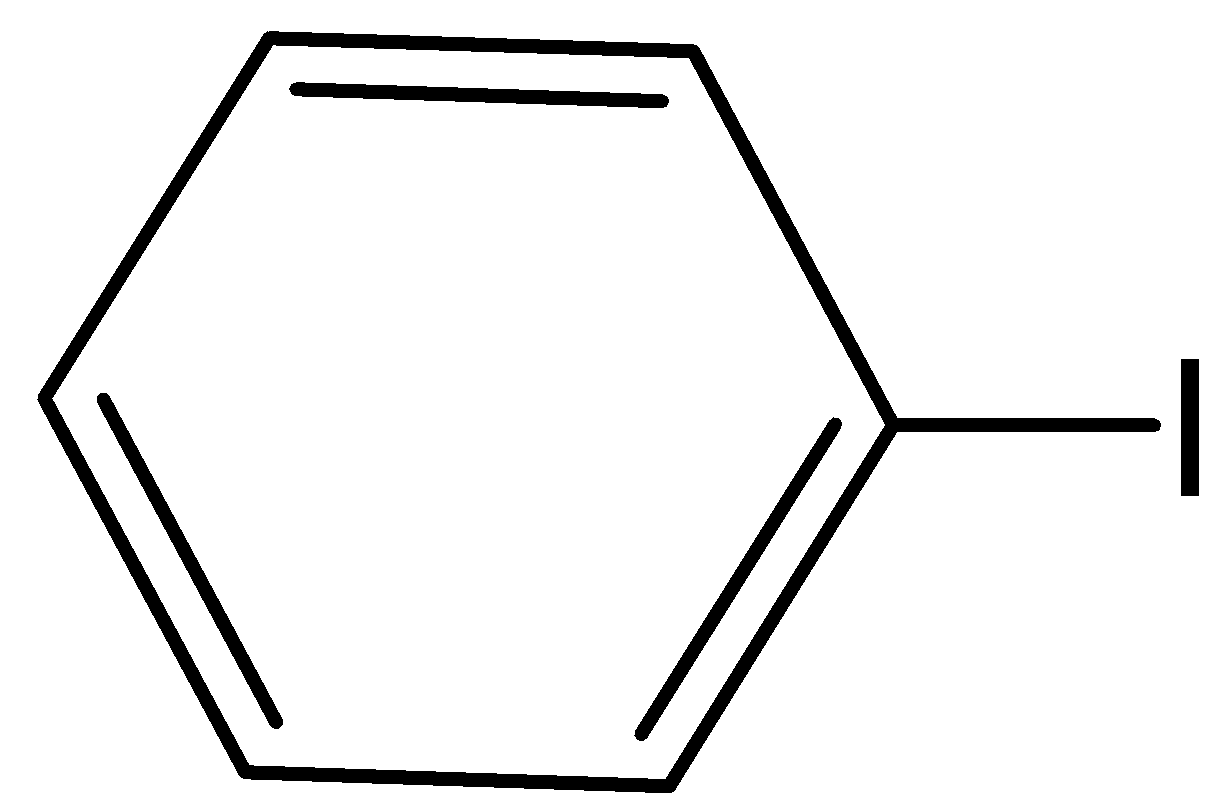
II
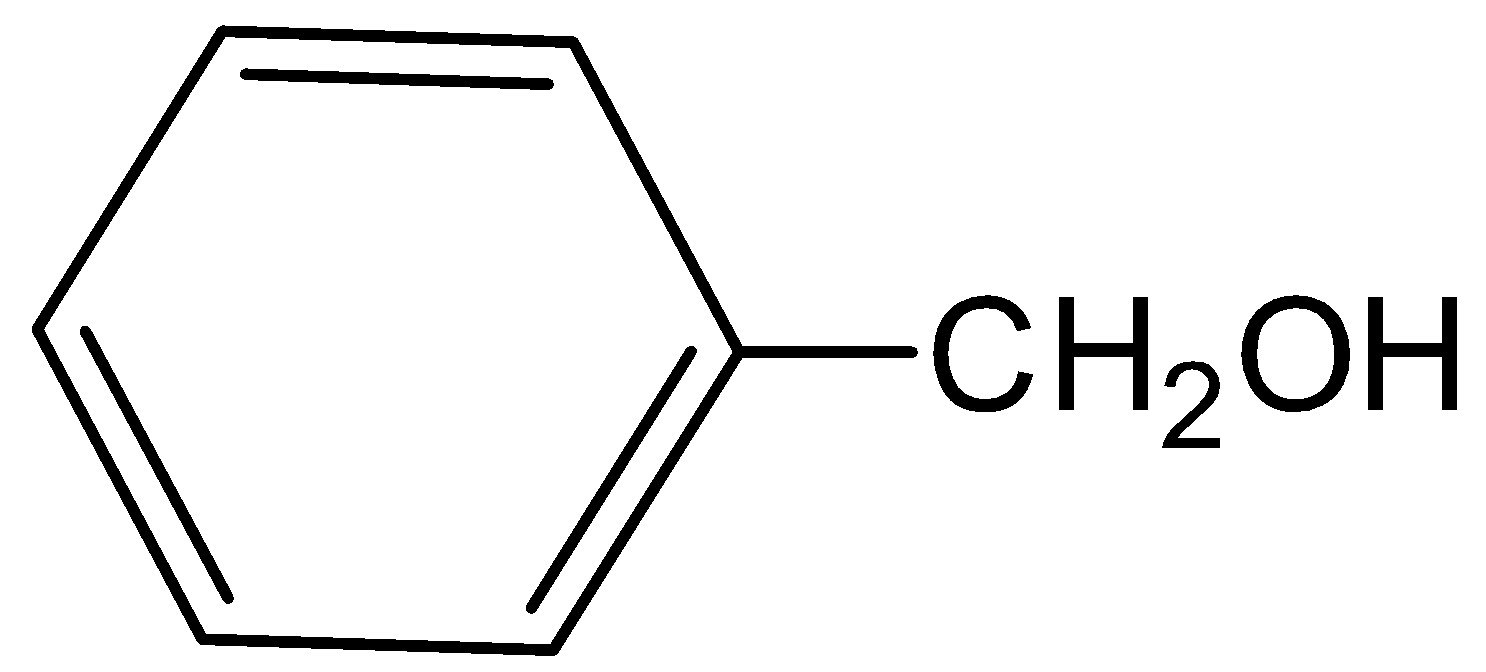
III
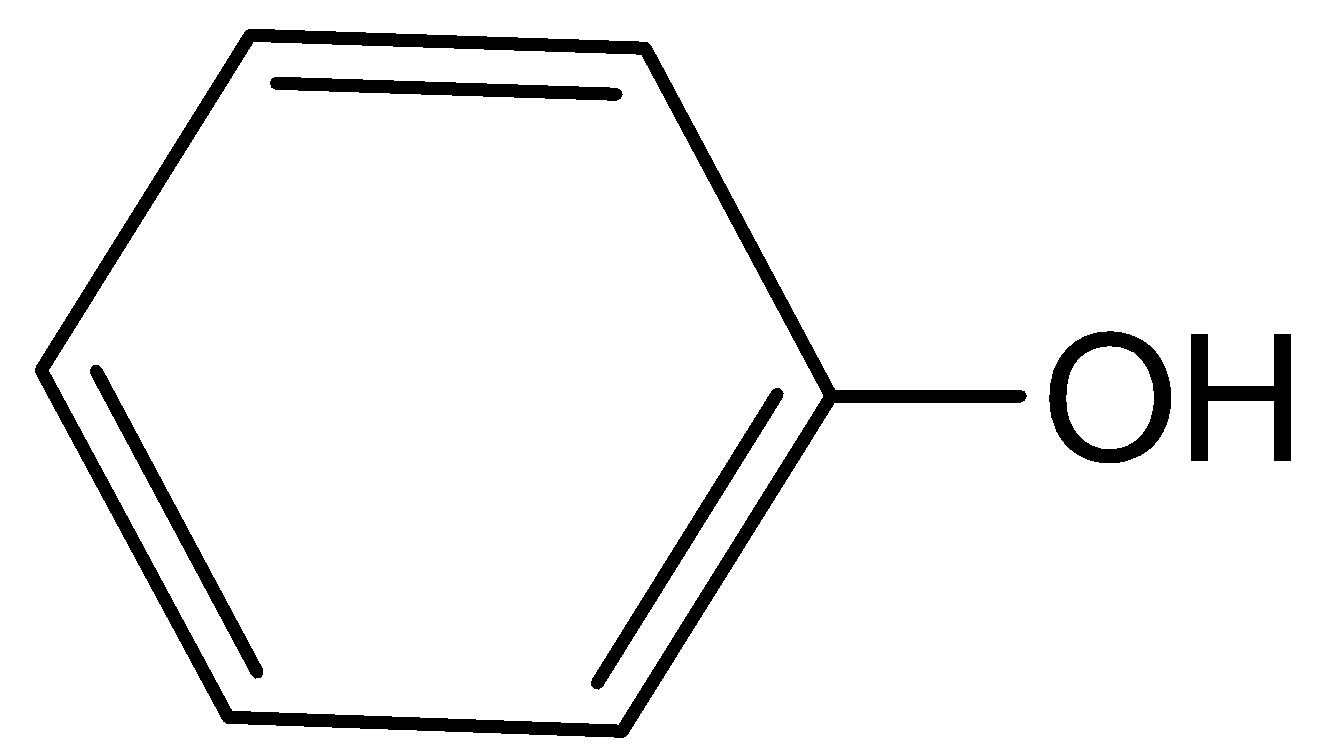
IV
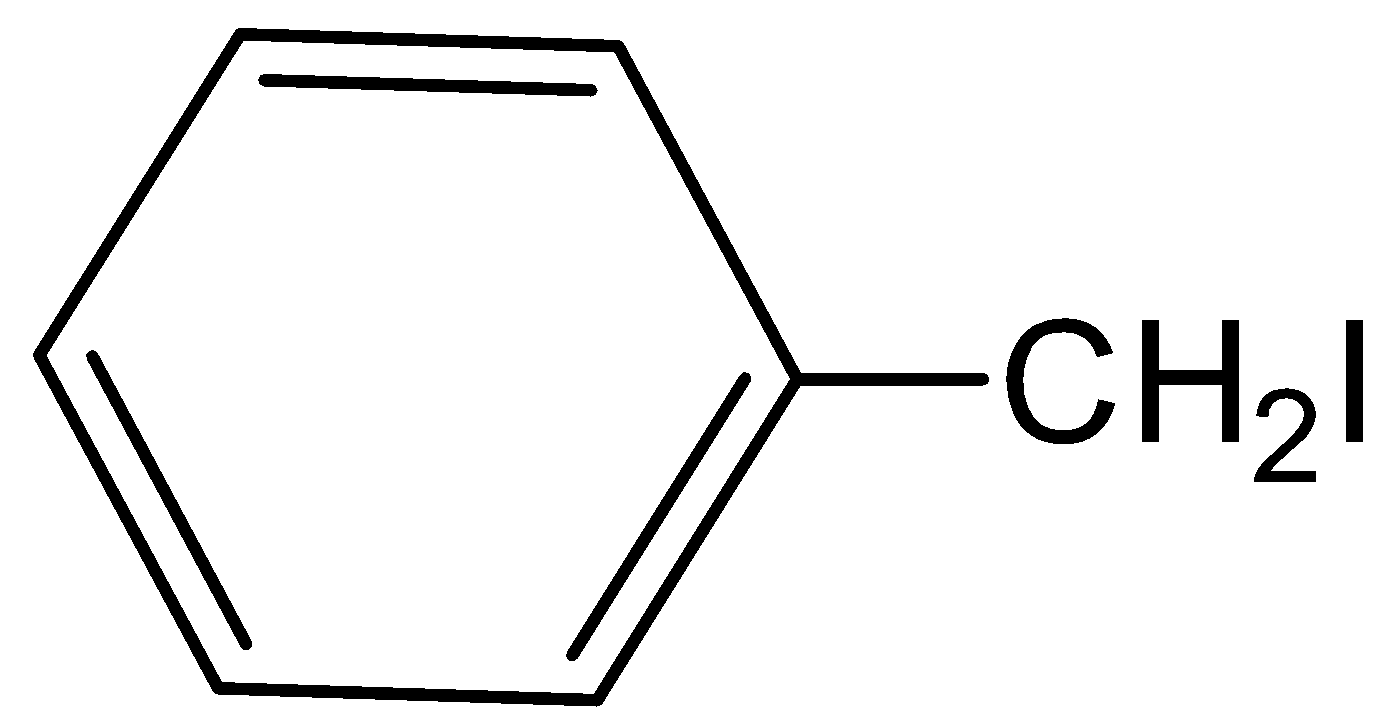
A.Both I and II
B.Both III and IV
C.Both I and III
D.Both II and III





Answer
510.9k+ views
Hint: We need to know that the benzyl phenyl ether is a chemical compound with ether as a functional group having the molecular formula \[{C_{13}}{H_{12}}O\]. Here, benzyl phenyl ether is reacted with hydrogen iodide and there is a formation of two products. The hydrogen iodide is also known as hydroiodic acid which acts as a strong acid.
Complete answer:
We have to remember that during the reaction of benzyl phenyl ether, there will not form any product with alcohol functional group and will not form iodo benzene. Hence, option (A) is incorrect.
Here, benzyl phenyl ether is reacting with hydrogen iodide and there is a formation of two products and that is phenol and benzyl iodide. And the reaction can be written as,
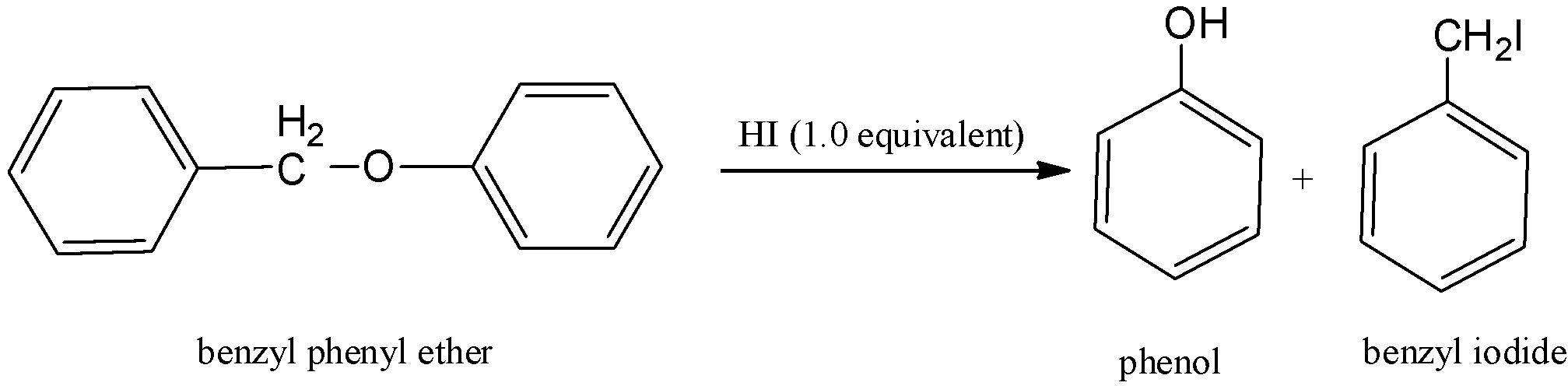
Therefore, Both III and IV are the major products, which means phenol and benzyl iodide. Hence, option (B) is correct.
When benzyl phenyl ether is reacting with hydrogen iodide, there will not form, benzyl iodide. Hence, option (C) is incorrect.
During this reaction, they will not form.Hence, the option (D) is incorrect
Hence, the option (B) is correct.
Note:
Here, ether group is reacted with hydrogen iodide and forms two products. Which is phenol and benzyl iodide. Phenol is an aromatic compound having the formula \[{C_6}{H_5}OH\]. Here the phenyl group is bonded with the hydroxyl group which is slightly acidic. And benzyl iodide is a chemical compound with formula \[{C_7}{H_7}I\]. Here, the iodide group is attached with the benzene group.
Complete answer:
We have to remember that during the reaction of benzyl phenyl ether, there will not form any product with alcohol functional group and will not form iodo benzene. Hence, option (A) is incorrect.
Here, benzyl phenyl ether is reacting with hydrogen iodide and there is a formation of two products and that is phenol and benzyl iodide. And the reaction can be written as,

Therefore, Both III and IV are the major products, which means phenol and benzyl iodide. Hence, option (B) is correct.
When benzyl phenyl ether is reacting with hydrogen iodide, there will not form, benzyl iodide. Hence, option (C) is incorrect.
During this reaction, they will not form.Hence, the option (D) is incorrect
Hence, the option (B) is correct.
Note:
Here, ether group is reacted with hydrogen iodide and forms two products. Which is phenol and benzyl iodide. Phenol is an aromatic compound having the formula \[{C_6}{H_5}OH\]. Here the phenyl group is bonded with the hydroxyl group which is slightly acidic. And benzyl iodide is a chemical compound with formula \[{C_7}{H_7}I\]. Here, the iodide group is attached with the benzene group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




