
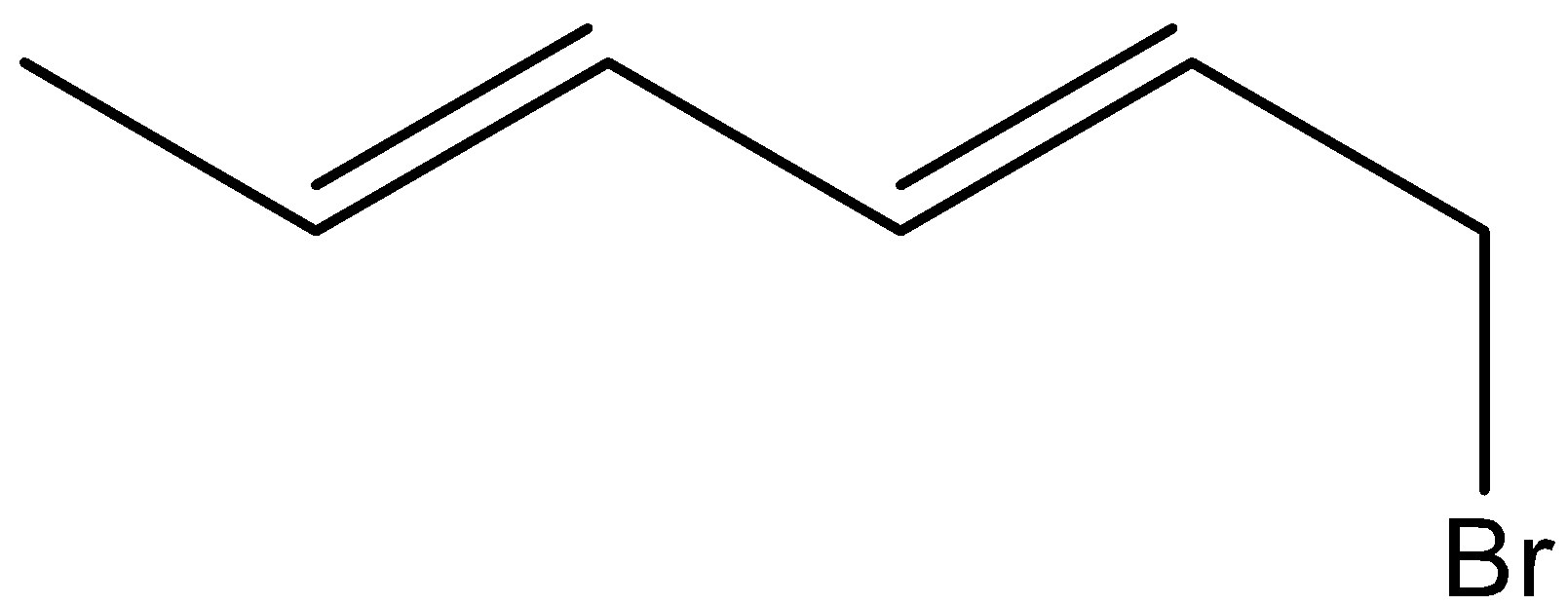
Major product is-
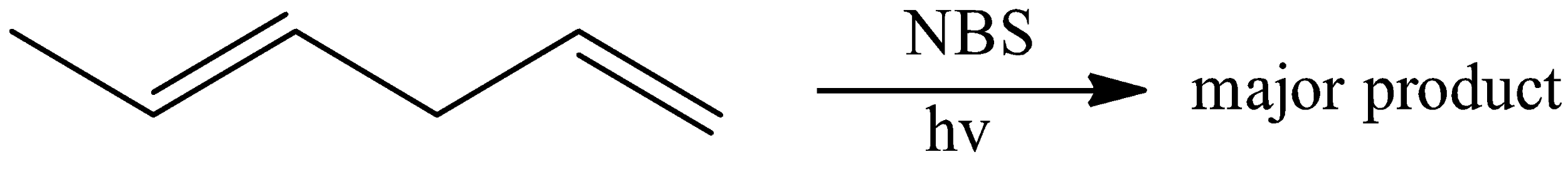
A.
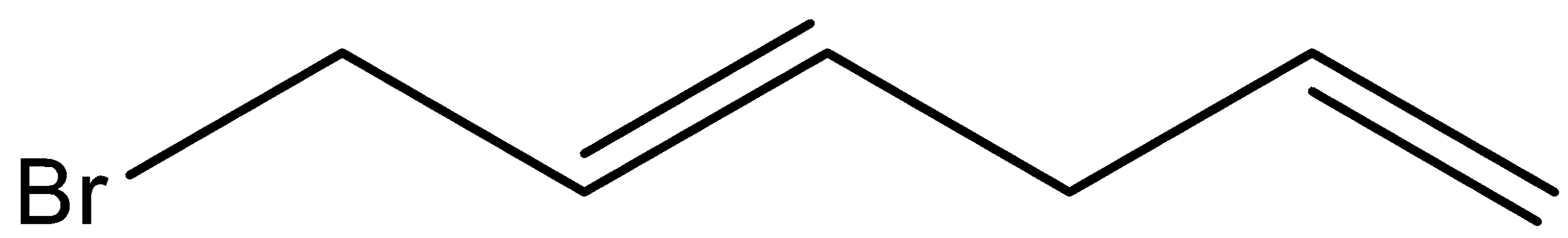
B.
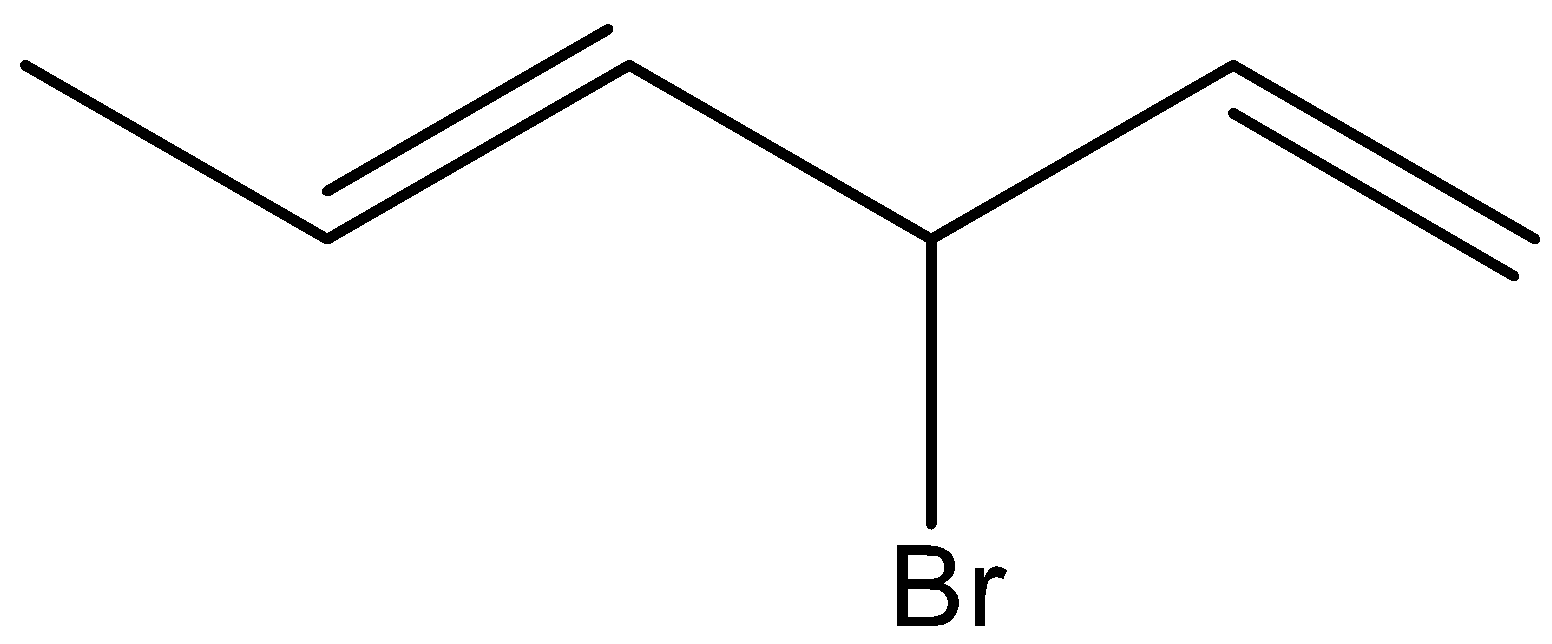
C.
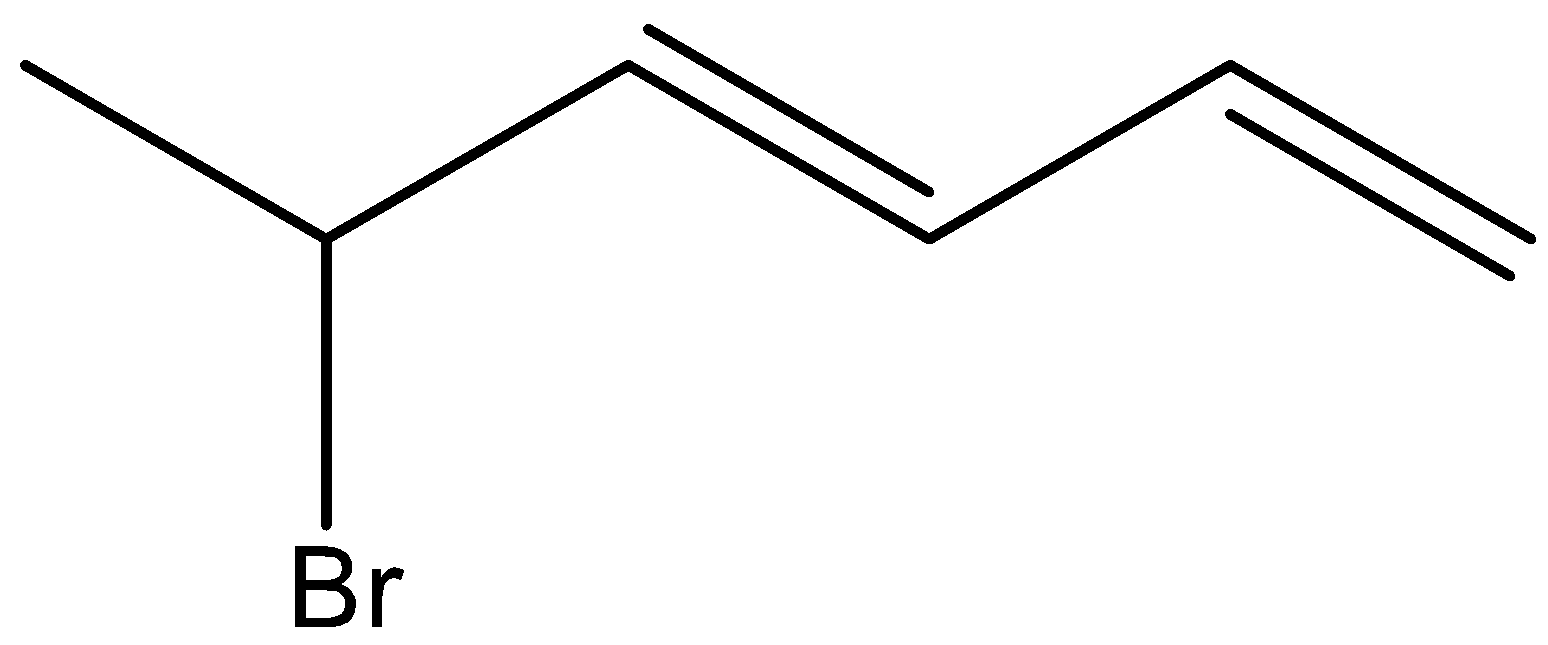
D.
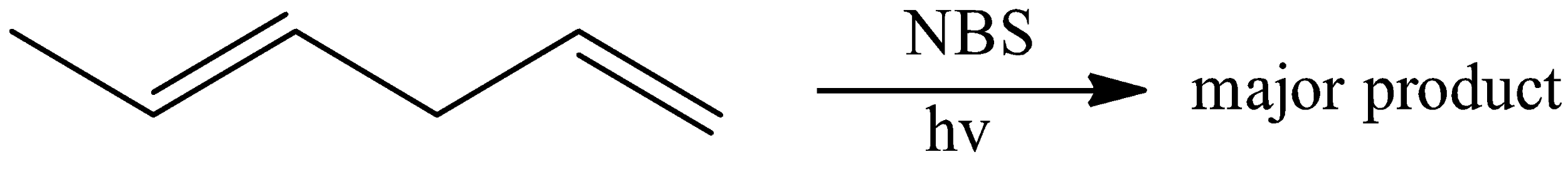




Answer
510.3k+ views
Hint: Here, the given reactant is (E)-hexta-1,4-diene which is reacted with NBS in the presence of light. NBS is a common reagent which is used for the allylic bromination reaction and its full form is N- bromosuccinimide. And the reaction takes place by the replacement of an allylic hydrogen atom which is present in the carbon atom adjacent to the double bond.
Complete answer:
When alkene is reacted with NBS reagent in the presence of heat, the allylic hydrogen present in the hydrogen is replaced by bromine present in NBS reagent. But, here it does not happen. Hence, option (A) is incorrect.
When an alkene is reacted with NBS reagent under heat, an allylic bromination reaction takes place. Which means, the hydrogen atom is replaced by bromine atom present in the NBS reagent. The bromine undergoes the hemolytic cleavage in the presence of light and it is the initiation reaction. And the abstraction of allylic hydrogen takes place in the propagation step and there is a formation of desired product.
And here, (E)- Hence, the option (B) is incorrect. 1,4- hexadiene is reacted with NBS reagent and there is a formation of (E)-3-bromohexa-1,4-diene as the major product. Let’s see the reaction,
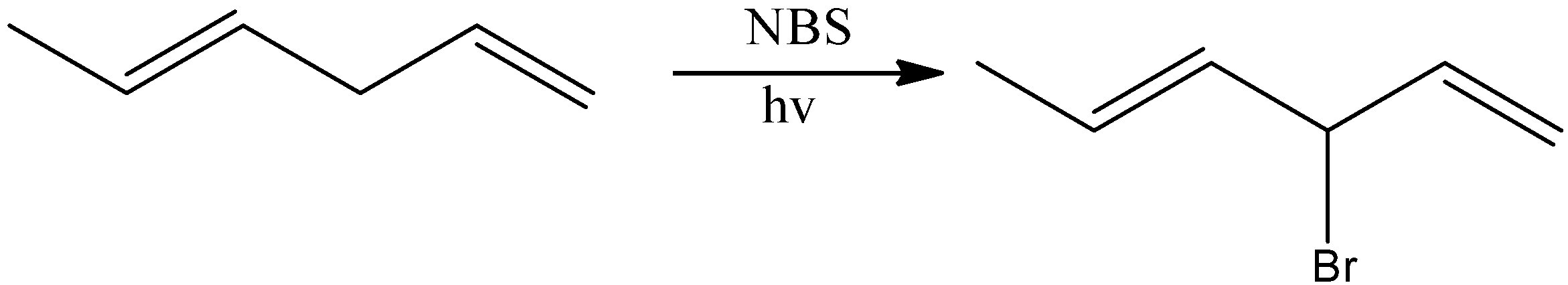
Hence, option (B) is correct.
Here, the allylic hydrogen is not replaced by bromine or NBS reagent. Hence, option (C) is incorrect.
(2E, 4E)-1-bromohexa-2, 4-diene is not the correct structure of a major product. Hence, the option (D) is incorrect.
Hence, option (B) is correct.
Note:
In the case of allylic bromination, the NBS reagent is used as a substitute for the bromine atom and this bromine is reacted with the double bond and there is a formation of dibromides. And if the alkene is reacted with NBS in the presence of light, there will get allyl bromide. And this allylic bromination undergoes a radical mechanism which is different from antimarkov nikov’s radical bromination.
Complete answer:
When alkene is reacted with NBS reagent in the presence of heat, the allylic hydrogen present in the hydrogen is replaced by bromine present in NBS reagent. But, here it does not happen. Hence, option (A) is incorrect.
When an alkene is reacted with NBS reagent under heat, an allylic bromination reaction takes place. Which means, the hydrogen atom is replaced by bromine atom present in the NBS reagent. The bromine undergoes the hemolytic cleavage in the presence of light and it is the initiation reaction. And the abstraction of allylic hydrogen takes place in the propagation step and there is a formation of desired product.
And here, (E)- Hence, the option (B) is incorrect. 1,4- hexadiene is reacted with NBS reagent and there is a formation of (E)-3-bromohexa-1,4-diene as the major product. Let’s see the reaction,

Hence, option (B) is correct.
Here, the allylic hydrogen is not replaced by bromine or NBS reagent. Hence, option (C) is incorrect.
(2E, 4E)-1-bromohexa-2, 4-diene is not the correct structure of a major product. Hence, the option (D) is incorrect.
Hence, option (B) is correct.
Note:
In the case of allylic bromination, the NBS reagent is used as a substitute for the bromine atom and this bromine is reacted with the double bond and there is a formation of dibromides. And if the alkene is reacted with NBS in the presence of light, there will get allyl bromide. And this allylic bromination undergoes a radical mechanism which is different from antimarkov nikov’s radical bromination.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




