
What is the major alkene formed in the following Hofmann elimination?
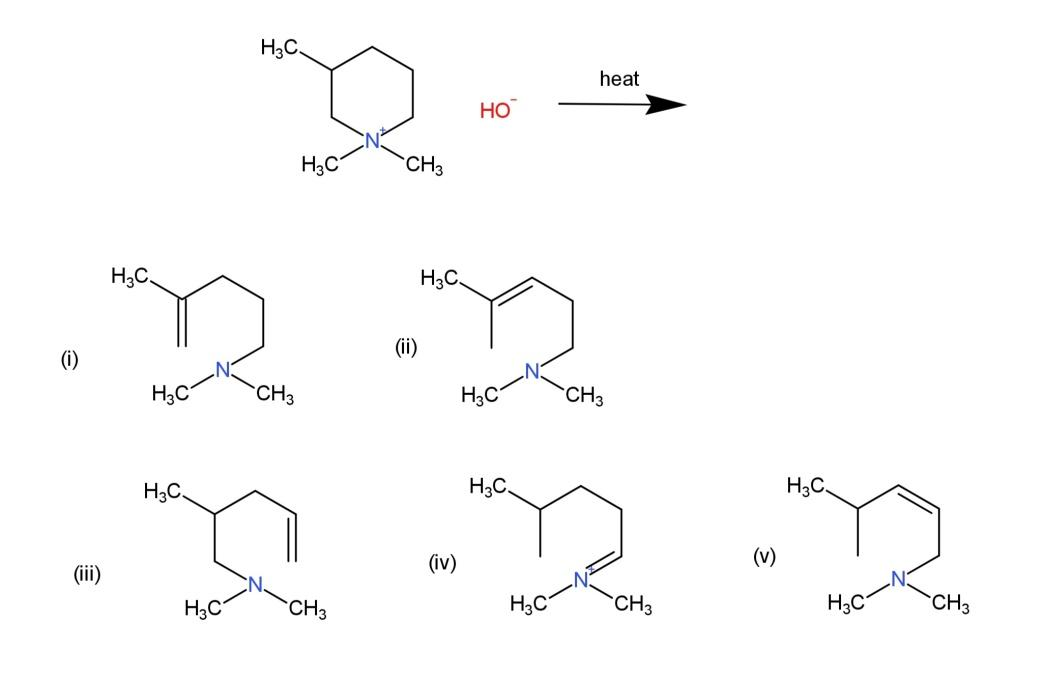
A. i
B. ii
C. iii
D. iv
E. v

Answer
534k+ views
Hint: Hofmann elimination is the reaction where an aliphatic or aromatic amine that has beta-hydrogen is converted to an unsaturated compound. It takes place in the presence of a base along with high temperature (heat).
Complete answer:
Hofmann elimination is the reaction where an amine reacts with a base to form an alkene. The amine consist of a $\beta $ - hydrogen whose removal marks the formation of a double bond. This elimination is based on the Saytzeff rule that states that the major product will be that product which is more stable.
The given compound when subjected to a base and heat, have the $\beta $ - hydrogen removed, after the removal of $\beta $ - hydrogen, the most stable carbocation will form the major product. The carbocation is stable when the saturation takes place on the $2{}^\circ $ carbocation, which is the product (i), while other products contain less stable $1{}^\circ $ carbocations. Therefore the reaction will be as follows:

Hence, the product formed is (i), so, option A is correct.
Note:
Hofmann elimination reaction and Hofmann bromamide degradation reactions are two different reactions, but both consist of a base as a reactant. Hofmann bromamide consists of the formation of aliphatic or aromatic amines from the reaction of amide, bromine and sodium hydroxide (base). Hofmann elimination is the preparation of unsaturated compounds.
Complete answer:
Hofmann elimination is the reaction where an amine reacts with a base to form an alkene. The amine consist of a $\beta $ - hydrogen whose removal marks the formation of a double bond. This elimination is based on the Saytzeff rule that states that the major product will be that product which is more stable.
The given compound when subjected to a base and heat, have the $\beta $ - hydrogen removed, after the removal of $\beta $ - hydrogen, the most stable carbocation will form the major product. The carbocation is stable when the saturation takes place on the $2{}^\circ $ carbocation, which is the product (i), while other products contain less stable $1{}^\circ $ carbocations. Therefore the reaction will be as follows:

Hence, the product formed is (i), so, option A is correct.
Note:
Hofmann elimination reaction and Hofmann bromamide degradation reactions are two different reactions, but both consist of a base as a reactant. Hofmann bromamide consists of the formation of aliphatic or aromatic amines from the reaction of amide, bromine and sodium hydroxide (base). Hofmann elimination is the preparation of unsaturated compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




