
Magnesium has \[12\] protons. How many electrons are in its first energy level of magnesium?
Answer
547.8k+ views
Hint:The electronic configuration says about how the electrons are distributed in atomic orbitals. The electronic configuration is filled according to the energy they have. They follow the standard notion in which electrons containing the atomic subshells are placed in a sequence. For example, the Magnesium’s electron configuration; \[Mg = {\text{ }}1{s^2}2{s^2}2{p^6}3{s^2}\]
Complete step-by-step answer: The full electronic configuration of any element is written in standard format. But when it comes to higher elements will consist of many numbers of orbital, so for simplification condensed electronic configuration is noted.
Suppose if we have considered iron as an example,
The full electronic configuration;
\[Fe = {\text{ }}1{s^2}2{s^2}2{p^6}3{s^2}\,3{p^6}\,4{s^2}\,3{d^6}\]
The condensed electron configuration;
\[Fe = {\text{ [Ar]}}\,{\text{4}}{s^2}3{d^6}\]
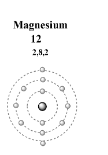
The magnesium is the \[{12^{th}}\] element in the periodic table. Its electronic configuration is as follows;
\[Mg = {\text{ }}1{s^2}2{s^2}2{p^6}3{s^2}\]
Whereas, in the condensed form of electronic configuration, the noble gas makes it easier to denote it.
So, let’s see how the noble gas configuration of magnesium will be;
\[Mg = {\text{ [Ne]}}\,3{s^2}\]
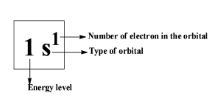
Let’s discuss about the energy levels;
As we know that hydrogen is having only one electron so the first energy level is having only one electron.
So, every atom except the hydrogen at the first energy level will be having two electrons.
So, the answer to this question is \[2\] . The magnesium has \[2\] electrons in its first energy level.
Therefore, the first energy level can only hold a maximum of \[2\] electrons because it only contains one orbital i.e., \[1s\] orbital.
Note:Magnesium \[(Mg)\] whose atomic number is \[12\] , which is having \[12\] electrons. The \[12\] protons are there in atomic nuclei. A neutral atom has the same number of protons as well as the same number of electrons.
When we write the condensed form or abbreviated form of the electronic configuration of magnesium, we have to start writing the noble gas configuration in the previous period, and then continue with the electronic configuration of the element in the new period. The noble gas symbol is enclosed in brackets. Then the next periods’ electronic configuration is added.
When it comes to the case of magnesium, it is in the \[{3^{rd}}\] period of the table, so that the noble gas will be argon, which is the noble gas in period \[2\] . Since the magnesium is in the group \[2\] and the electronic configuration will be ending with\[3s\] sublevels.
Complete step-by-step answer: The full electronic configuration of any element is written in standard format. But when it comes to higher elements will consist of many numbers of orbital, so for simplification condensed electronic configuration is noted.

Suppose if we have considered iron as an example,
The full electronic configuration;
\[Fe = {\text{ }}1{s^2}2{s^2}2{p^6}3{s^2}\,3{p^6}\,4{s^2}\,3{d^6}\]
The condensed electron configuration;
\[Fe = {\text{ [Ar]}}\,{\text{4}}{s^2}3{d^6}\]

The magnesium is the \[{12^{th}}\] element in the periodic table. Its electronic configuration is as follows;
\[Mg = {\text{ }}1{s^2}2{s^2}2{p^6}3{s^2}\]
Whereas, in the condensed form of electronic configuration, the noble gas makes it easier to denote it.
So, let’s see how the noble gas configuration of magnesium will be;
\[Mg = {\text{ [Ne]}}\,3{s^2}\]
Let’s discuss about the energy levels;
As we know that hydrogen is having only one electron so the first energy level is having only one electron.
So, every atom except the hydrogen at the first energy level will be having two electrons.
So, the answer to this question is \[2\] . The magnesium has \[2\] electrons in its first energy level.
Therefore, the first energy level can only hold a maximum of \[2\] electrons because it only contains one orbital i.e., \[1s\] orbital.
Note:Magnesium \[(Mg)\] whose atomic number is \[12\] , which is having \[12\] electrons. The \[12\] protons are there in atomic nuclei. A neutral atom has the same number of protons as well as the same number of electrons.
When we write the condensed form or abbreviated form of the electronic configuration of magnesium, we have to start writing the noble gas configuration in the previous period, and then continue with the electronic configuration of the element in the new period. The noble gas symbol is enclosed in brackets. Then the next periods’ electronic configuration is added.
When it comes to the case of magnesium, it is in the \[{3^{rd}}\] period of the table, so that the noble gas will be argon, which is the noble gas in period \[2\] . Since the magnesium is in the group \[2\] and the electronic configuration will be ending with\[3s\] sublevels.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




