
LPG used as domestic fuel is mixed with a strong-smelling liquid for---------.
(A) Better combustion
(B) Good small/fragrance
(C) Detection of gas leakage
(D) Higher calorific value
Answer
589.2k+ views
Hint: Generally a fuel gas does not have any odour. LPG is a mixture of propane and butane and it does not have any odour. By adding a strong-smelling liquid called Ethyl Mercaptan LPG gives a strong smell of rotten cabbages.
Complete step by step solution:
-Generally fuel gases like LPG supplied for domestic use do not have an odour.
-If there is any leak of LPG we cannot find it because we cannot see the gases and there is no odour to LPG.
-So, to detect the leakage of LPG gas a strong-smelling chemical called Ethyl Mercaptan is added.
-After adding Ethyl Mercaptan to LPG we can detect the leakage very easily.
-So, the correct option is C, detection of gas leakage.
Additional information:
-Ethyl Mercaptan is a colourless gas and has a low boiling point.
-The molecular formula of Ethyl Mercaptan is\[{{C}_{2}}{{H}_{6}}S\].
-Ethyl Mercaptan is also called Ethanethiol.
-The structure of Ethyl Mercaptan is as follows.
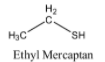
-Due to the presence of sulphur Ethyl Mercaptan is too smelly.
-Generally Sulphur compounds are smelly in nature.
Note:-Ethyl Mercaptan is only added to LPG gas (used for the domestic purpose), not to CNG, which is used to run vehicles.
LPG – Liquefied Petroleum Gas
CNG – Compressed Natural Gas (composed of methane gas)
-By adding Ethyl Mercaptan to LPG the calorific value of LPG is not going to affect.
Complete step by step solution:
-Generally fuel gases like LPG supplied for domestic use do not have an odour.
-If there is any leak of LPG we cannot find it because we cannot see the gases and there is no odour to LPG.
-So, to detect the leakage of LPG gas a strong-smelling chemical called Ethyl Mercaptan is added.
-After adding Ethyl Mercaptan to LPG we can detect the leakage very easily.
-So, the correct option is C, detection of gas leakage.
Additional information:
-Ethyl Mercaptan is a colourless gas and has a low boiling point.
-The molecular formula of Ethyl Mercaptan is\[{{C}_{2}}{{H}_{6}}S\].
-Ethyl Mercaptan is also called Ethanethiol.
-The structure of Ethyl Mercaptan is as follows.

-Due to the presence of sulphur Ethyl Mercaptan is too smelly.
-Generally Sulphur compounds are smelly in nature.
Note:-Ethyl Mercaptan is only added to LPG gas (used for the domestic purpose), not to CNG, which is used to run vehicles.
LPG – Liquefied Petroleum Gas
CNG – Compressed Natural Gas (composed of methane gas)
-By adding Ethyl Mercaptan to LPG the calorific value of LPG is not going to affect.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




