
When liquid water is cooled, it contracts until a temperature of approximately \[4\] degrees Celsius is reached. After that it expands slightly until it reaches the freezing point.
Reason-
The distance between the H-O bond is more than O-O attraction in the water. Hence, the water freezing over into ice is held together not by the O-O attraction but the H-O attraction.
A.Both Assertion and Reason are correct and the Reason is the correct explanation of assertion.
B.Both Assertion and reason are correct but Reason is not a good explanation for assertion.
C.Assertion is correct but reason is wrong.
D.Both assertion and reason are incorrect.
Answer
591.6k+ views
Hint: Water has an abnormal property where it expands when temperature goes from ${4^ \circ }$ Celsius to ${0^ \circ }$ Celsius whereas other liquids contract on cooling. The water has maximum density at \[4\] degrees Celsius but it becomes less dense on ${0^ \circ }$ Celsius.
Complete step by step answer:
-Here the given assertion(A) is “When liquid water is cooled, it contracts until a temperature of approximately \[4\] degrees Celsius is reached. After that it expands slightly until it reaches the freezing point”.
-And the given reason is “The distance between the H-O bond is more than O-O attraction in the water. Hence, the water freezing over into ice is held together not by the O-O attraction but the H-O attraction”.
-Water contracts on cooling until the temperature reaches approximately \[4\] degrees Celsius. Then water starts expanding slightly till it reaches the freezing point.
At freezing point, the tendency of hydrogen to form a network making H-O bond increases.
- As the temperature decreases this tendency starts increasing and water forms a very open structure with big holes.
-As extra strong bonds can be easily made, water releases energy.
-But still it takes more space and ice expands on freezing.
This open structure is the cause of ice being less dense than water like this-
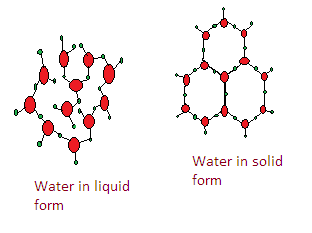
So the assertion and reason both are correct.
Hence the correct answer is A.
Additional information:
This property of water is very useful in nature because if the water behaved normally like other liquids then many water bodies like lakes, ponds would freeze in winter and kill all the marine life residing inside the water.
Note:
The properties of water are-
-Water is colourless, odourless and tasteless liquid.
-It freezes at ${0^ \circ }$ Celsius and boils at ${100^ \circ }$ Celsius under one atm pressure.
-It cannot conduct heat and electricity.
-Water is neutral hence does not give only colour on litmus paper.
Complete step by step answer:
-Here the given assertion(A) is “When liquid water is cooled, it contracts until a temperature of approximately \[4\] degrees Celsius is reached. After that it expands slightly until it reaches the freezing point”.
-And the given reason is “The distance between the H-O bond is more than O-O attraction in the water. Hence, the water freezing over into ice is held together not by the O-O attraction but the H-O attraction”.
-Water contracts on cooling until the temperature reaches approximately \[4\] degrees Celsius. Then water starts expanding slightly till it reaches the freezing point.
At freezing point, the tendency of hydrogen to form a network making H-O bond increases.
- As the temperature decreases this tendency starts increasing and water forms a very open structure with big holes.
-As extra strong bonds can be easily made, water releases energy.
-But still it takes more space and ice expands on freezing.
This open structure is the cause of ice being less dense than water like this-

So the assertion and reason both are correct.
Hence the correct answer is A.
Additional information:
This property of water is very useful in nature because if the water behaved normally like other liquids then many water bodies like lakes, ponds would freeze in winter and kill all the marine life residing inside the water.
Note:
The properties of water are-
-Water is colourless, odourless and tasteless liquid.
-It freezes at ${0^ \circ }$ Celsius and boils at ${100^ \circ }$ Celsius under one atm pressure.
-It cannot conduct heat and electricity.
-Water is neutral hence does not give only colour on litmus paper.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




