
Liquid $S{O_2}$ is aprotic solvent. If true, write \[1\] , else write \[0\].
Answer
556.5k+ views
Hint: We must remember that the solvent which does not have $O - H$ or $N - H$ bonds are called an aprotic solvent. Aprotic solvent, protic means hydrogen atoms and a means without. The specified definition of aprotic solvent is that the molecules do not contain hydrogen atoms on oxygen or nitrogen, i.e., molecules can’t form hydrogen bonds with themselves, however they may accept hydrogen from new molecules.
Complete step by step answer:
We must need to know that the solvents often utilized in chemistry are characterised by their physical characteristics. Among the foremost vital are whether or not they’re protic or aprotic. Solvents are typically as a liquid that has the ability to dissolve, suspend or extract different materials.
Aprotic solvent: A solvent which is never a hydrogen bond donor.
Protic solvent: A solvent that’s a hydrogen bond donor
We need to remember that the sulphur dioxide \[\left( {S{O_2}} \right)\] (also known as sulphurous anhydride or sulphur $\left( {IV} \right)$ oxide) is toxic gas.
We know that the liquid Sulphur dioxide produced from gas $S{O_2}$, concentration within range of $1\% - 100\% $ using different processes.
Structure of Sulphur dioxide is,
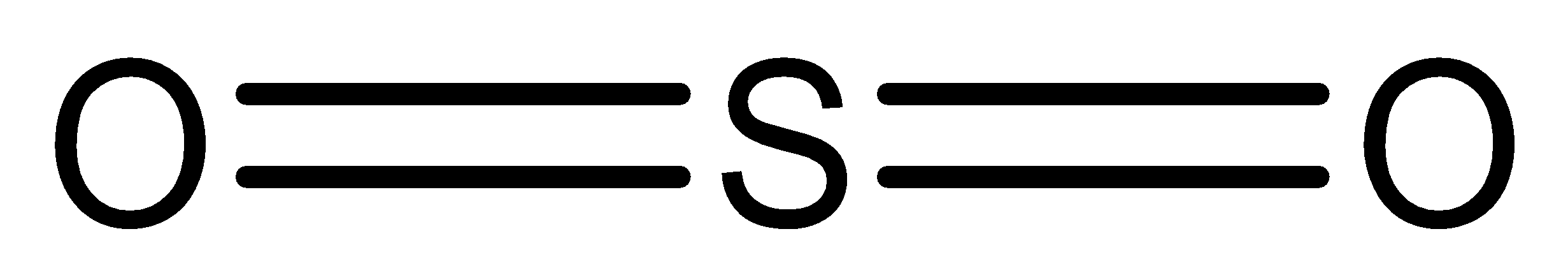
We must remember that the sulphur dioxide is an aprotic solvent due to it doesn’t containing $O - H$ or $N - H$ groups.
$S{O_2}$ can’t form $H - $bonds because there’s no hydrogen. The bonds are polar because the Sulphur is somewhat positive and the oxygen is slightly negative, so there’s some intermolecular bonding happening, however without hydrogen, they’re no hydrogen bonds.
\[1\]- Liquid $S{O_2}$ is aprotic solvent.
Therefore, option B is the correct answer and it is an anti-aromatic.
Note:
We must need to remember that highly oxidizing salts are dissolved by Sulphur dioxide, a versatile inert solvent. $S{O_2}$ is a source of the sulfonyl group in organic synthesis, used occasionally. For the production of liquid Sulphur dioxide, several different processes are partial condensation, compression and condensing, absorption and acidification and from Sulphur trioxide and Sulphur.
Complete step by step answer:
We must need to know that the solvents often utilized in chemistry are characterised by their physical characteristics. Among the foremost vital are whether or not they’re protic or aprotic. Solvents are typically as a liquid that has the ability to dissolve, suspend or extract different materials.
Aprotic solvent: A solvent which is never a hydrogen bond donor.
Protic solvent: A solvent that’s a hydrogen bond donor
We need to remember that the sulphur dioxide \[\left( {S{O_2}} \right)\] (also known as sulphurous anhydride or sulphur $\left( {IV} \right)$ oxide) is toxic gas.
We know that the liquid Sulphur dioxide produced from gas $S{O_2}$, concentration within range of $1\% - 100\% $ using different processes.
Structure of Sulphur dioxide is,
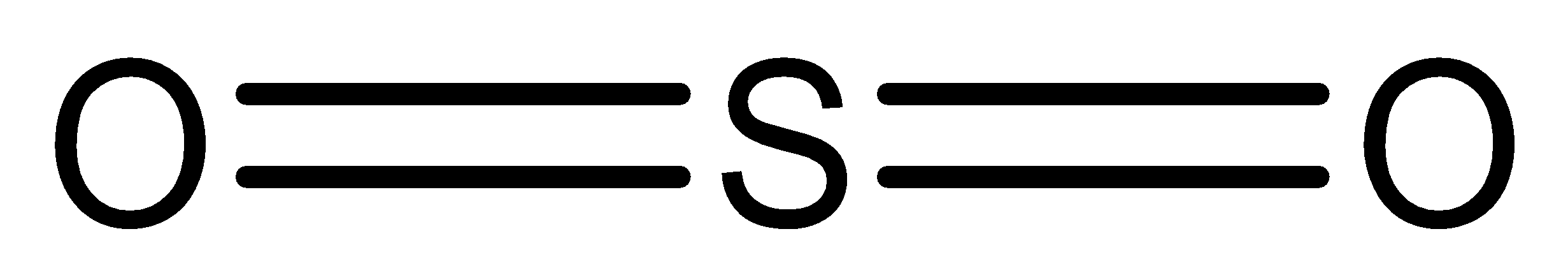
We must remember that the sulphur dioxide is an aprotic solvent due to it doesn’t containing $O - H$ or $N - H$ groups.
$S{O_2}$ can’t form $H - $bonds because there’s no hydrogen. The bonds are polar because the Sulphur is somewhat positive and the oxygen is slightly negative, so there’s some intermolecular bonding happening, however without hydrogen, they’re no hydrogen bonds.
\[1\]- Liquid $S{O_2}$ is aprotic solvent.
Therefore, option B is the correct answer and it is an anti-aromatic.
Note:
We must need to remember that highly oxidizing salts are dissolved by Sulphur dioxide, a versatile inert solvent. $S{O_2}$ is a source of the sulfonyl group in organic synthesis, used occasionally. For the production of liquid Sulphur dioxide, several different processes are partial condensation, compression and condensing, absorption and acidification and from Sulphur trioxide and Sulphur.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




