
Lewis structure of \[{\rm{Be}}{{\rm{F}}_{\rm{2}}}\]
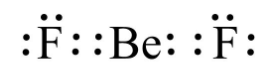
(A) True
(B) False
Answer
577.2k+ views
Hint: As we know that, Lewis acids are electron deficient compounds which do not have complete octet. Beryllium is the element of alkaline earth metal and fluorine is the element of the halogen family.
Complete solution
Lewis represented the atom as positively charged kernel and the outer shell. The outermost shell is occupied by the eight electrons. Thus, these electrons in the outermost shell take part in chemical combinations in Lewis structure. Lewis structures are represented by Lewis symbols or electron dot symbols. In this structure of \[{\rm{Be}}{{\rm{F}}_{\rm{2}}}\], valence electrons are represented by electron dot symbols.
As we know that, the beryllium atom is the member of the second group in the periodic table and it has two electrons in its outermost shell. Therefore, these electrons only participate in bond formation with fluorine. So, one electron of each fluorine forms bonds with two electrons of beryllium.
This is shown below
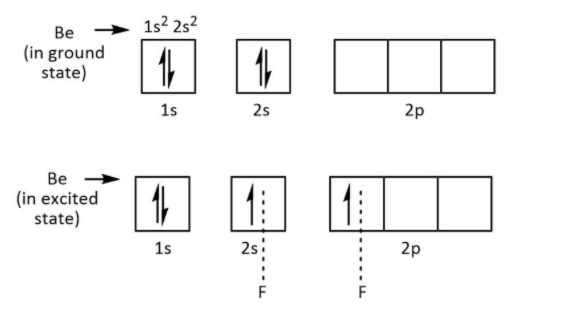
So only four electrons are available in the structure of \[{\rm{Be}}{{\rm{F}}_{\rm{2}}}\], it needs four electrons to complete its octet. That’s why \[{\rm{Be}}{{\rm{F}}_{\rm{2}}}\] also known as Lewis acid.
The actual structure is represented as-
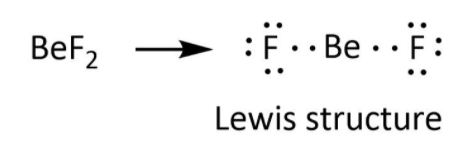
Therefore, the correct option for the given statement is option (B) that is False.
Note:
The above structure is the exception of Lewis octet rule, in which octet is incomplete but there are several molecules or compounds which have more than eight electrons in the outermost shell of central atom such as \[{\rm{S}}{{\rm{F}}_{\rm{6}}}\] has twelve electrons around the central atom, \[{\rm{P}}{{\rm{F}}_5}\] has ten and \[{\rm{I}}{{\rm{F}}_7}\] has fourteen electrons. These molecules are stable.
Complete solution
Lewis represented the atom as positively charged kernel and the outer shell. The outermost shell is occupied by the eight electrons. Thus, these electrons in the outermost shell take part in chemical combinations in Lewis structure. Lewis structures are represented by Lewis symbols or electron dot symbols. In this structure of \[{\rm{Be}}{{\rm{F}}_{\rm{2}}}\], valence electrons are represented by electron dot symbols.
As we know that, the beryllium atom is the member of the second group in the periodic table and it has two electrons in its outermost shell. Therefore, these electrons only participate in bond formation with fluorine. So, one electron of each fluorine forms bonds with two electrons of beryllium.
This is shown below

So only four electrons are available in the structure of \[{\rm{Be}}{{\rm{F}}_{\rm{2}}}\], it needs four electrons to complete its octet. That’s why \[{\rm{Be}}{{\rm{F}}_{\rm{2}}}\] also known as Lewis acid.
The actual structure is represented as-
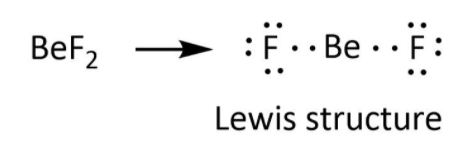
Therefore, the correct option for the given statement is option (B) that is False.
Note:
The above structure is the exception of Lewis octet rule, in which octet is incomplete but there are several molecules or compounds which have more than eight electrons in the outermost shell of central atom such as \[{\rm{S}}{{\rm{F}}_{\rm{6}}}\] has twelve electrons around the central atom, \[{\rm{P}}{{\rm{F}}_5}\] has ten and \[{\rm{I}}{{\rm{F}}_7}\] has fourteen electrons. These molecules are stable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




