
What is the Lewis structure for the resonance form of \[Cl{O_2}^ - \]?
Answer
503.1k+ views
Hint: Lewis structures are the diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that can exist in the molecule. It is found that these are called electron dot structures. It basically adds up two lines between the atoms that show shared pairs.
Complete answer:
Chlorine dioxide has an odd number of valence electrons and is considered a paramagnetic radical. The compound is composed of a central chlorine atom because chlorine is less electronegative than oxygen. The two oxygen atoms connected via covalent bonds.
Chlorine dioxide has two resonance structures with a double bond and three electrons on the other. Both resonance structures have a bent molecular geometry with an $O = Cl - O$ bond angle of $117.6^\circ $.
Every atom has a formal charge; we can reduce the number of formal charges by moving a lone pair of electrons from oxygen to form a $Cl = O$ double bond. This gives us two new structures in which one oxygen atom has a formal charge $ - 1$ and the other atom has a formal charge of $0$.
The two resonance structure of \[Cl{O_2}^ - \] are:
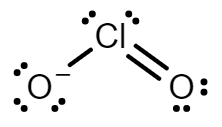
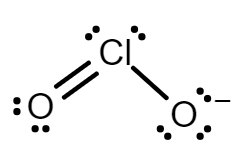
Note:
Lewis structure mainly describes the structure of molecules with the help of symbols for the chemical species and also dot symbols. It is found that octet rule exists because the atoms of most of the elements become more stable by attaining the electronic configuration of an element.
Complete answer:
Chlorine dioxide has an odd number of valence electrons and is considered a paramagnetic radical. The compound is composed of a central chlorine atom because chlorine is less electronegative than oxygen. The two oxygen atoms connected via covalent bonds.
Chlorine dioxide has two resonance structures with a double bond and three electrons on the other. Both resonance structures have a bent molecular geometry with an $O = Cl - O$ bond angle of $117.6^\circ $.
Every atom has a formal charge; we can reduce the number of formal charges by moving a lone pair of electrons from oxygen to form a $Cl = O$ double bond. This gives us two new structures in which one oxygen atom has a formal charge $ - 1$ and the other atom has a formal charge of $0$.
The two resonance structure of \[Cl{O_2}^ - \] are:


Note:
Lewis structure mainly describes the structure of molecules with the help of symbols for the chemical species and also dot symbols. It is found that octet rule exists because the atoms of most of the elements become more stable by attaining the electronic configuration of an element.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




