
What is the Lewis formula for carbon oxy-sulphide $ \left( COS \right) $ ?
Answer
521.4k+ views
Hint :We know that for solving this problem, you should have the knowledge about polyatomic ion and oxidation number. Polyatomic ion is an ion containing more than one atom.Oxidation number is the number of electrons lost or gained by an element during a reaction.
Complete Step By Step Answer:
Lewis structure helps in representing valence electrons of a molecule. On calculating the formal
Charges with respect to the Lewis structure, the charge on a polyatomic ion can be identified easily. Let's first try to draw the Lewis structure of trichloride. It has three chlorine atoms and only one boron so boron will be a central atom in this molecular structure.
Step-1: To solve the given problem, let’s determine the valence electron of all the atoms present in the compound.
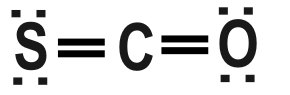
Step-2: Here, the compound is Carbon Oxy Sulphide. Here, Carbon has $ 4 $ valence electron, Oxygen contains
$ 6 $ valence electron and sulphur also contains $ 6 $ valence electron.
Step-3: If we look closely the formula can have the same Lewis structure as that of $ C{{O}_{2}} $ . Here carbon have $ 4 $ valence electron and $ 2 $ Oxygen atoms have $ 6 $ valence electron which can be compared with
$ 1 $ Oxygen valence and $ 1 $ Sulphur valence of $ COS. $
So, keeping the structure of COS similar to $ C{{O}_{2}} $ and replacing $ 1 $ Oxygen to Sulphur we get the
answer.
Note :
Remember that while determining the Lewis structure of a compound, the valence electron should be remembered and determined correctly so as to avoid any mistakes and if possible the Lewis structures of some basic compounds can be learned to simplify the process. VSEPR is valence shell electron pair repulsion, it simply tells us that nonbonding and bonding electron pairs of the central atom in a molecule push each other away.
Complete Step By Step Answer:
Lewis structure helps in representing valence electrons of a molecule. On calculating the formal
Charges with respect to the Lewis structure, the charge on a polyatomic ion can be identified easily. Let's first try to draw the Lewis structure of trichloride. It has three chlorine atoms and only one boron so boron will be a central atom in this molecular structure.
Step-1: To solve the given problem, let’s determine the valence electron of all the atoms present in the compound.
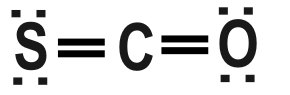
Step-2: Here, the compound is Carbon Oxy Sulphide. Here, Carbon has $ 4 $ valence electron, Oxygen contains
$ 6 $ valence electron and sulphur also contains $ 6 $ valence electron.
Step-3: If we look closely the formula can have the same Lewis structure as that of $ C{{O}_{2}} $ . Here carbon have $ 4 $ valence electron and $ 2 $ Oxygen atoms have $ 6 $ valence electron which can be compared with
$ 1 $ Oxygen valence and $ 1 $ Sulphur valence of $ COS. $
So, keeping the structure of COS similar to $ C{{O}_{2}} $ and replacing $ 1 $ Oxygen to Sulphur we get the
answer.
Note :
Remember that while determining the Lewis structure of a compound, the valence electron should be remembered and determined correctly so as to avoid any mistakes and if possible the Lewis structures of some basic compounds can be learned to simplify the process. VSEPR is valence shell electron pair repulsion, it simply tells us that nonbonding and bonding electron pairs of the central atom in a molecule push each other away.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




