
What is the Lewis dot diagram for carbon?
Answer
535.5k+ views
Hint: The valence electrons of the atoms can be represented using the Lewis dot diagram. They are a simplified version of the valence shell electrons of a molecule and use dots to represent the valence electrons.
Complete answer:
To draw the Lewis dot structure of carbon, the following steps should be followed.
- We first need to find out the number of electrons in the atom.
Since the atomic number of carbon is 12 and is in the ground state, the number of electrons in the carbon atom is 12.
- Then electronic configuration of the element must be written. This will help us figure out how many electrons are there in the outermost shell.
\[C^{12}:1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}\]
- Now, we determine the number of electrons in the outermost shell to find out the number of valence electrons.
Here we can see that the outermost shell is the 2nd shell for which the principal quantum number n=2.
The 2nd shell of carbon has 4 electrons, 2 from s subshell and 2 from p subshell. Hence the carbon atom has 4 valence electrons.
- Now, the valence electrons are depicted on the symbol of the atom to draw the Lewis dot diagram.
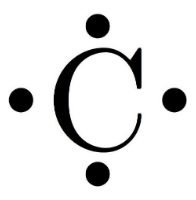
Note: It should be noted that when the atom is an ion, the electrons added to or subtracted from the Lewis dot diagram are equal to the magnitude of the charge. In the case of an anion (negative charge), electrons are added and in the case of a cation (positive) electrons are subtracted.
Complete answer:
To draw the Lewis dot structure of carbon, the following steps should be followed.
- We first need to find out the number of electrons in the atom.
Since the atomic number of carbon is 12 and is in the ground state, the number of electrons in the carbon atom is 12.
- Then electronic configuration of the element must be written. This will help us figure out how many electrons are there in the outermost shell.
\[C^{12}:1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}\]
- Now, we determine the number of electrons in the outermost shell to find out the number of valence electrons.
Here we can see that the outermost shell is the 2nd shell for which the principal quantum number n=2.
The 2nd shell of carbon has 4 electrons, 2 from s subshell and 2 from p subshell. Hence the carbon atom has 4 valence electrons.
- Now, the valence electrons are depicted on the symbol of the atom to draw the Lewis dot diagram.

Note: It should be noted that when the atom is an ion, the electrons added to or subtracted from the Lewis dot diagram are equal to the magnitude of the charge. In the case of an anion (negative charge), electrons are added and in the case of a cation (positive) electrons are subtracted.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




