
Label the following distributed ring as cis or trans and as (a, a); (e, e) or (a, e).
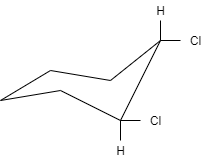
A.Trans (a, e)
B.Cis (a, e)
C.Cis (e, e)
D.Trans (a, e)

Answer
568.2k+ views
Hint: Cis and trans isomers are the geometric isomers where trans isomers are more stable than cis isomers. Stereo isomers are defined as the isomers that have the same molecular formula but have different orientations of atoms.
Complete step by step answer:
In this question, the conformation of cyclohexane is chair conformation, which is the most stable conformation and has the least energy. Cyclohexane shows different kinds of conformation which includes boat, twist, half-chair and chair conformation.
Chair conformation consists of twelve hydrogen atoms that are not structurally equivalent. The six of them lie on the periphery of the carbon ring, which is known as equatorial and the other six are located above and below the plane of the ring, that are known as axial. They are known as axial because they are located parallel to the symmetry axis of the ring. All the twelve hydrogens consist of 50% axial and 50% equatorial character.
In this question, the cyclohexane consists of two chlorine atoms that are located on the opposite side of the ring. Therefore, the structure is called trans. Both the chlorine atoms are located in equatorial positions. Therefore, they are represented as (e, e).
Hence, the disubstituted ring is labeled as trans (e, e). so, the correct option is (A).
Note: To identify cis and trans isomers, if two substituent groups are attached on the same side of the double bond then it is known as cis isomer and if two substituent groups are attached on the opposite side of the double bond then it is known as trans isomer.
-The cis isomers are polar molecules whereas the trans isomers are non-polar molecules. In cis isomers, the substituent groups are on the same side, therefore, one side of the molecule will have slightly positive charge, whereas other side will have slightly negative charge, which makes the molecule polar.
-The trans isomers are more stable than cis isomers because of the steric hindrance on cis isomers.
-In conclusion, the chlorine atoms are located on the equatorial position and show trans behavior therefore, the disubstituted ring is labeled as trans (e, e).
Complete step by step answer:
In this question, the conformation of cyclohexane is chair conformation, which is the most stable conformation and has the least energy. Cyclohexane shows different kinds of conformation which includes boat, twist, half-chair and chair conformation.
Chair conformation consists of twelve hydrogen atoms that are not structurally equivalent. The six of them lie on the periphery of the carbon ring, which is known as equatorial and the other six are located above and below the plane of the ring, that are known as axial. They are known as axial because they are located parallel to the symmetry axis of the ring. All the twelve hydrogens consist of 50% axial and 50% equatorial character.
In this question, the cyclohexane consists of two chlorine atoms that are located on the opposite side of the ring. Therefore, the structure is called trans. Both the chlorine atoms are located in equatorial positions. Therefore, they are represented as (e, e).
Hence, the disubstituted ring is labeled as trans (e, e). so, the correct option is (A).
Note: To identify cis and trans isomers, if two substituent groups are attached on the same side of the double bond then it is known as cis isomer and if two substituent groups are attached on the opposite side of the double bond then it is known as trans isomer.
-The cis isomers are polar molecules whereas the trans isomers are non-polar molecules. In cis isomers, the substituent groups are on the same side, therefore, one side of the molecule will have slightly positive charge, whereas other side will have slightly negative charge, which makes the molecule polar.
-The trans isomers are more stable than cis isomers because of the steric hindrance on cis isomers.
-In conclusion, the chlorine atoms are located on the equatorial position and show trans behavior therefore, the disubstituted ring is labeled as trans (e, e).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




