
What kind of bond would the electron dot formula for ${{\text{O}}_{\text{2}}}$ show?
Answer
557.4k+ views
Hint: We know that there are three modes of chemical combination, that is, by electron transfer and by sharing electrons. The chemical bond formed by sharing electrons is termed as covalent bond and when electron transfer results in chemical bond, then the chemical bond is termed as ionic bond.
Complete step by step answer:
Let’s first draw the electron dot formula of ${{\text{O}}_{\text{2}}}$. We know that the atomic number of oxygen is 8. The number of valence electrons is 6. As there are two oxygen atoms, they share two electrons from each other to achieve stable configuration. As the two electrons are shared between the oxygen atoms, formation of a double bond will take place.
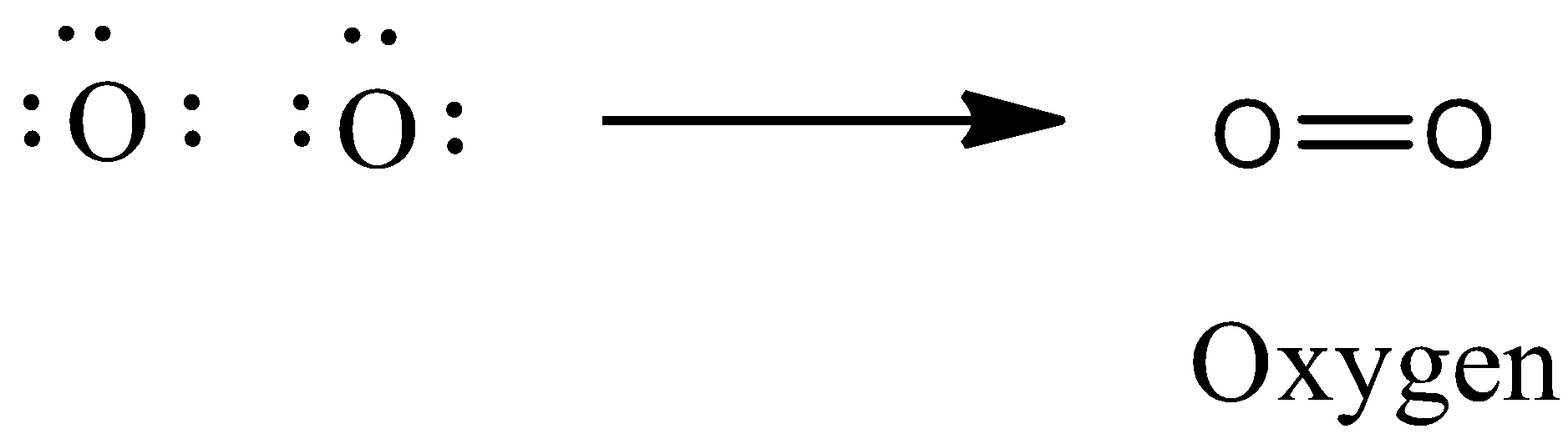
So, the kind of bond forms in ${{\text{O}}_{\text{2}}}$ is a double covalent bond.
Additional Information:
Let’s discuss ionic compounds in detail. An ionic bond is formed as a result of complete transference of one or more electrons from one atom to the other. This type of bond is found between metals and nonmetals. For example, NaCl is an ionic compound. Here, sodium is a metal and chlorine is a non-metal. Let’s discuss the formation of NaCl molecules. We know sodium is a metal and it has one valence electron and chlorine is a non-metal and has seven valence electrons. To achieve stable configuration, sodium loses its valence electron and chlorine gains this electron to complete its octet. In this way, NaCl forms.
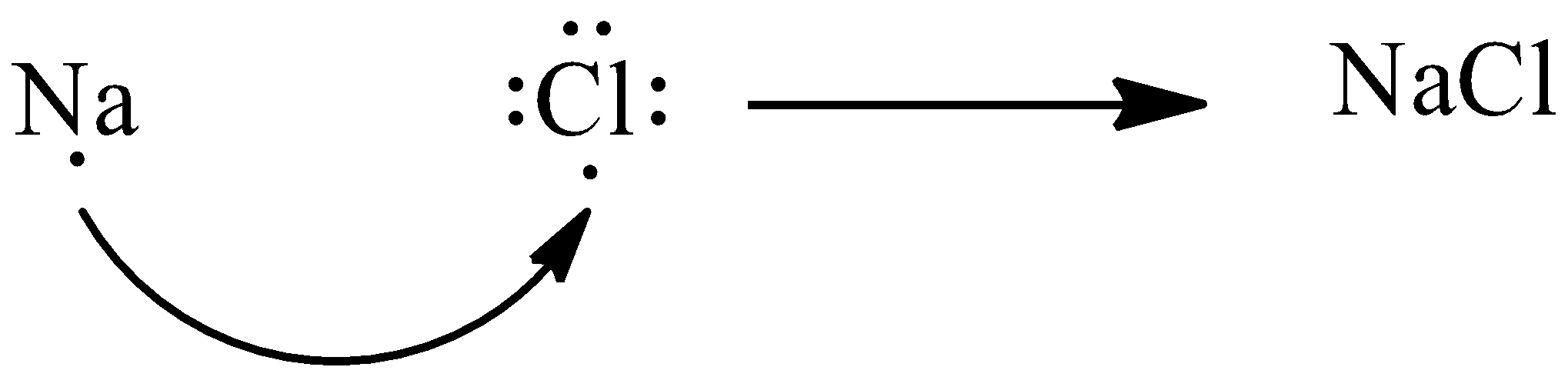
Note: It is to be noted that covalent bonds are always formed between two nonmetals, such as hydrogen molecule, oxygen molecule etc. There are three types of covalent bonds namely, single bond, double bond and triple bond.
Complete step by step answer:
Let’s first draw the electron dot formula of ${{\text{O}}_{\text{2}}}$. We know that the atomic number of oxygen is 8. The number of valence electrons is 6. As there are two oxygen atoms, they share two electrons from each other to achieve stable configuration. As the two electrons are shared between the oxygen atoms, formation of a double bond will take place.

So, the kind of bond forms in ${{\text{O}}_{\text{2}}}$ is a double covalent bond.
Additional Information:
Let’s discuss ionic compounds in detail. An ionic bond is formed as a result of complete transference of one or more electrons from one atom to the other. This type of bond is found between metals and nonmetals. For example, NaCl is an ionic compound. Here, sodium is a metal and chlorine is a non-metal. Let’s discuss the formation of NaCl molecules. We know sodium is a metal and it has one valence electron and chlorine is a non-metal and has seven valence electrons. To achieve stable configuration, sodium loses its valence electron and chlorine gains this electron to complete its octet. In this way, NaCl forms.
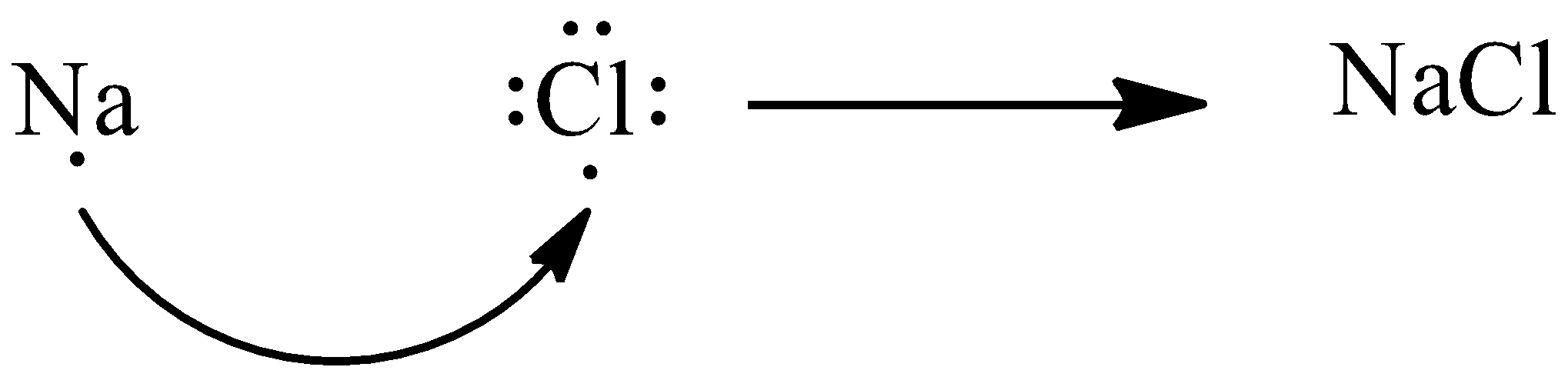
Note: It is to be noted that covalent bonds are always formed between two nonmetals, such as hydrogen molecule, oxygen molecule etc. There are three types of covalent bonds namely, single bond, double bond and triple bond.
Recently Updated Pages
Master Class 8 Social Science: Engaging Questions & Answers for Success

Master Class 8 English: Engaging Questions & Answers for Success

Class 8 Question and Answer - Your Ultimate Solutions Guide

Master Class 8 Maths: Engaging Questions & Answers for Success

Master Class 8 Science: Engaging Questions & Answers for Success

Master Class 7 English: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest States of India?

What is the theme or message of the poem The road not class 9 english CBSE

What are the major achievements of the UNO class 9 social science CBSE

Explain the importance of pH in everyday life class 9 chemistry CBSE

Differentiate between parenchyma collenchyma and sclerenchyma class 9 biology CBSE

Give 5 examples of refraction of light in daily life




