
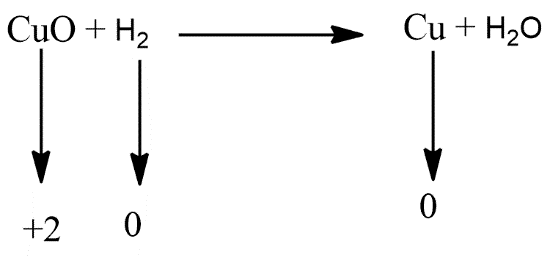
Justify the following reaction is redox reaction

Answer
515.1k+ views
Hint: Redox reaction can be defined as that chemical reaction in which electrons are transferred between the reactants participating in the chemical reaction. The transfer of electrons involves the changes in the oxidation state of the reacting species.
Complete answer:
The oxidation number also known as oxidation state which describes the degree of oxidation i.e. loss of electrons of an atom in a chemical compound. Conceptually the oxidation state may be positive, negative or zero.
Oxidation is defined as the loss of electrons or we can say that increase in the oxidation state of an atom, ion or molecule while reduction can be defined as the gain of electrons or decreases in the oxidation state of any given atom, ion or molecule.
Hence for a reaction to be of redox oxidation and reduction should occur in the same reaction i.e. one will act as a reducing agent and other one act like oxidizing agent. Here in the given reaction copper loses its two electrons and hydrogen gains its two electrons which implies that both oxidation and reduction takes place simultaneously in this reaction.
Thus this is said to be a redox reaction.
Note:
The increase in oxidation state of an atom, through a chemical reaction, is known as an oxidation; a decrease in oxidation state is known as a reduction. Such reactions involve the formal transfer of electrons: a net gain in electrons being a reduction, and a net loss of electrons being an oxidation. For pure elements, the oxidation state is zero.
Complete answer:
The oxidation number also known as oxidation state which describes the degree of oxidation i.e. loss of electrons of an atom in a chemical compound. Conceptually the oxidation state may be positive, negative or zero.
Oxidation is defined as the loss of electrons or we can say that increase in the oxidation state of an atom, ion or molecule while reduction can be defined as the gain of electrons or decreases in the oxidation state of any given atom, ion or molecule.
Hence for a reaction to be of redox oxidation and reduction should occur in the same reaction i.e. one will act as a reducing agent and other one act like oxidizing agent. Here in the given reaction copper loses its two electrons and hydrogen gains its two electrons which implies that both oxidation and reduction takes place simultaneously in this reaction.
Thus this is said to be a redox reaction.
Note:
The increase in oxidation state of an atom, through a chemical reaction, is known as an oxidation; a decrease in oxidation state is known as a reduction. Such reactions involve the formal transfer of electrons: a net gain in electrons being a reduction, and a net loss of electrons being an oxidation. For pure elements, the oxidation state is zero.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




