
i.Write molecular formula of Propane.
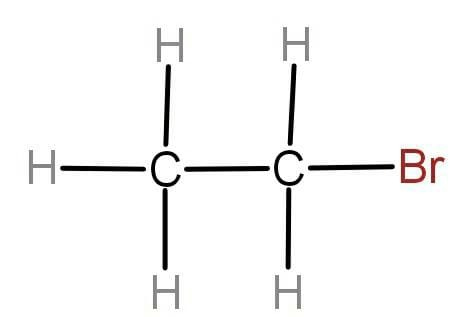
ii.Write the IUPAC name of

Answer
569.1k+ views
Hint: The Prefix Prop means the molecule has 3 carbon atoms. The suffix “ane” represents that the molecule only contains only single bonds. For 2 carbons the prefix used is “eth”.
Complete step by step answer:
i.Propane contains 3 carbon atoms and is saturated hydrocarbon that means it contains only a single bond. The general formula for the alkanes is: \[{{\text{C}}_{\text{n}}}{{\text{H}}_{2{\text{n}} + 2}}\].
Here n denotes the number of atoms of carbon present. In propane there are 3 carbon atoms and hence we will put the value of n as 3 in general formula to get the molecular formula as:
\[{{\text{C}}_3}{{\text{H}}_{2 \times 3 + 2}}\]. Hence we will get the molecular formula of propane as: \[{{\text{C}}_3}{{\text{H}}_8}\].
ii.
To write the IUPAC name we will first check whether the given molecule is saturated or unsaturated. The molecule contains all the single bonds that means the given molecule is alkane. If there are double and triple bonds present then the molecule will be unsaturated. Now we will look at the side substituent or functional group attached, in our question the side substituent is bromine which is named as Bromo which has writing names. Now we will check the number of carbon present in the chain that is 2 in our case.
The prefix used for the 2 carbon atoms is “eth” and for saturated carbon we use “ane”. Hence the IUPAC name for the given organic molecule is Bromoethane.
Note:
Bromoethane is a type of haloalkane. They are formed when one hydrogen atom is substituted with halogen. This mechanism involves the formation of carbocation. IUPAC stands for international union for pure and applied chemistry.
Complete step by step answer:
i.Propane contains 3 carbon atoms and is saturated hydrocarbon that means it contains only a single bond. The general formula for the alkanes is: \[{{\text{C}}_{\text{n}}}{{\text{H}}_{2{\text{n}} + 2}}\].
Here n denotes the number of atoms of carbon present. In propane there are 3 carbon atoms and hence we will put the value of n as 3 in general formula to get the molecular formula as:
\[{{\text{C}}_3}{{\text{H}}_{2 \times 3 + 2}}\]. Hence we will get the molecular formula of propane as: \[{{\text{C}}_3}{{\text{H}}_8}\].
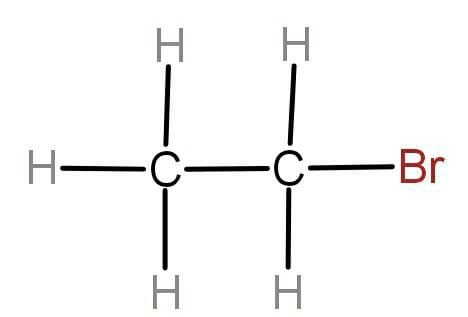
ii.

To write the IUPAC name we will first check whether the given molecule is saturated or unsaturated. The molecule contains all the single bonds that means the given molecule is alkane. If there are double and triple bonds present then the molecule will be unsaturated. Now we will look at the side substituent or functional group attached, in our question the side substituent is bromine which is named as Bromo which has writing names. Now we will check the number of carbon present in the chain that is 2 in our case.
The prefix used for the 2 carbon atoms is “eth” and for saturated carbon we use “ane”. Hence the IUPAC name for the given organic molecule is Bromoethane.
Note:
Bromoethane is a type of haloalkane. They are formed when one hydrogen atom is substituted with halogen. This mechanism involves the formation of carbocation. IUPAC stands for international union for pure and applied chemistry.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




