
What is the IUPAC Name of this compound?
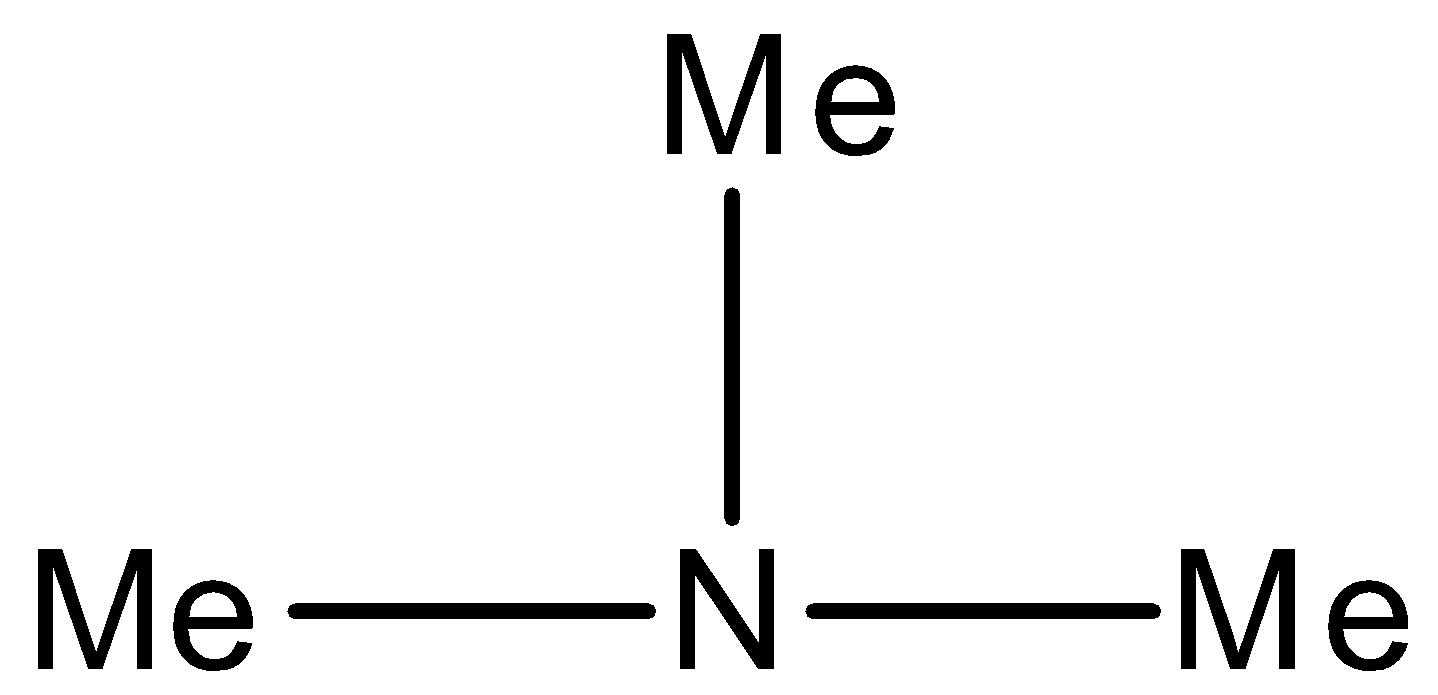
A) Trimethylamine
B) N-Dimethylmethanamine
C) 3-methylamine
D) None of the above

Answer
524.1k+ views
Hint: We need to know that it is easy to identify the IUPAC name for the compound while looking at the formula if we know the functional groups, like
\[N{H_2}\]: Amine
\[OH\] : Alcohol
\[COOH\]: Acid and so on.
Complete step by step answer:
We will move option wise to solve this question:
Option A) this option is incorrect as while looking at the structure it is right to say it as trimethylamine as it has 3 methyl groups and an amine group. But in the question it is asked to give an IUPAC Name which is not trimethylamine.
Option B) This option is correct as the IUPAC name for this compound is N-Dimethylmethanamine. We can draw the structure of N-Dimethylmethanamine as,
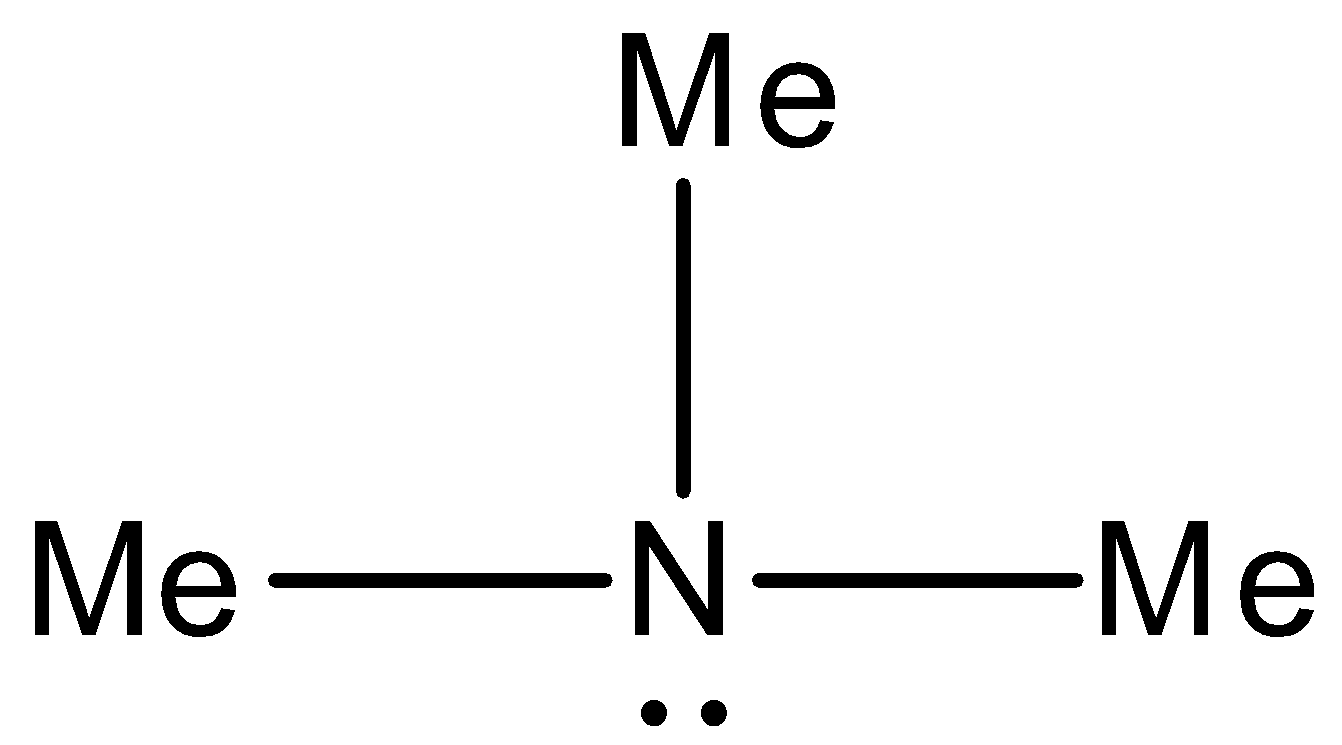
This can be seen from the structure \[N - Me\] is a methenamine group and as it has left with two methyl groups thus IUPAC name for this compound is N-Dimethylmethanamine.
Option C) this is an incorrect option as this nomenclature is not used to define either amine or methyl groups.
Option D) this is an incorrect option as we get option B as the correct answer.
Hence, the correct answer is, ‘Option D’.
Note: We need to know that it is a tertiary amine having a lone pair of electrons therefore it works as a good nucleophile in most of the reactions. We have to remember that the methylamine is an organic compound having a chemical formula as \[\left( {C{H_3}} \right)N{H_2}\]. We need to know that the methylamine has a pungent fishy odor and it appears to be a colourless gas or a liquid.
\[N{H_2}\]: Amine
\[OH\] : Alcohol
\[COOH\]: Acid and so on.
Complete step by step answer:
We will move option wise to solve this question:
Option A) this option is incorrect as while looking at the structure it is right to say it as trimethylamine as it has 3 methyl groups and an amine group. But in the question it is asked to give an IUPAC Name which is not trimethylamine.
Option B) This option is correct as the IUPAC name for this compound is N-Dimethylmethanamine. We can draw the structure of N-Dimethylmethanamine as,

This can be seen from the structure \[N - Me\] is a methenamine group and as it has left with two methyl groups thus IUPAC name for this compound is N-Dimethylmethanamine.
Option C) this is an incorrect option as this nomenclature is not used to define either amine or methyl groups.
Option D) this is an incorrect option as we get option B as the correct answer.
Hence, the correct answer is, ‘Option D’.
Note: We need to know that it is a tertiary amine having a lone pair of electrons therefore it works as a good nucleophile in most of the reactions. We have to remember that the methylamine is an organic compound having a chemical formula as \[\left( {C{H_3}} \right)N{H_2}\]. We need to know that the methylamine has a pungent fishy odor and it appears to be a colourless gas or a liquid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




