
What is the IUPAC name of the given compound?
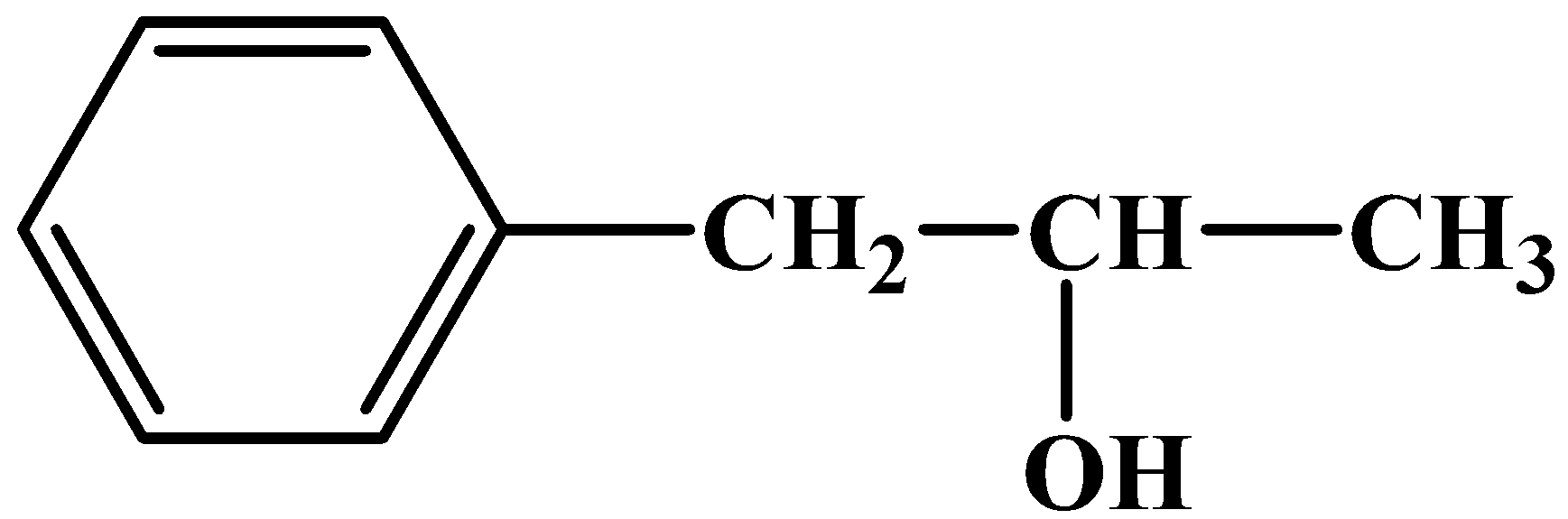
(a).$1-Phenylpropan-2-ol$
(b).$3-Phenylpropan-2-ol$
(c).$2-hydroxypropanobenzene$
(d.) None of these
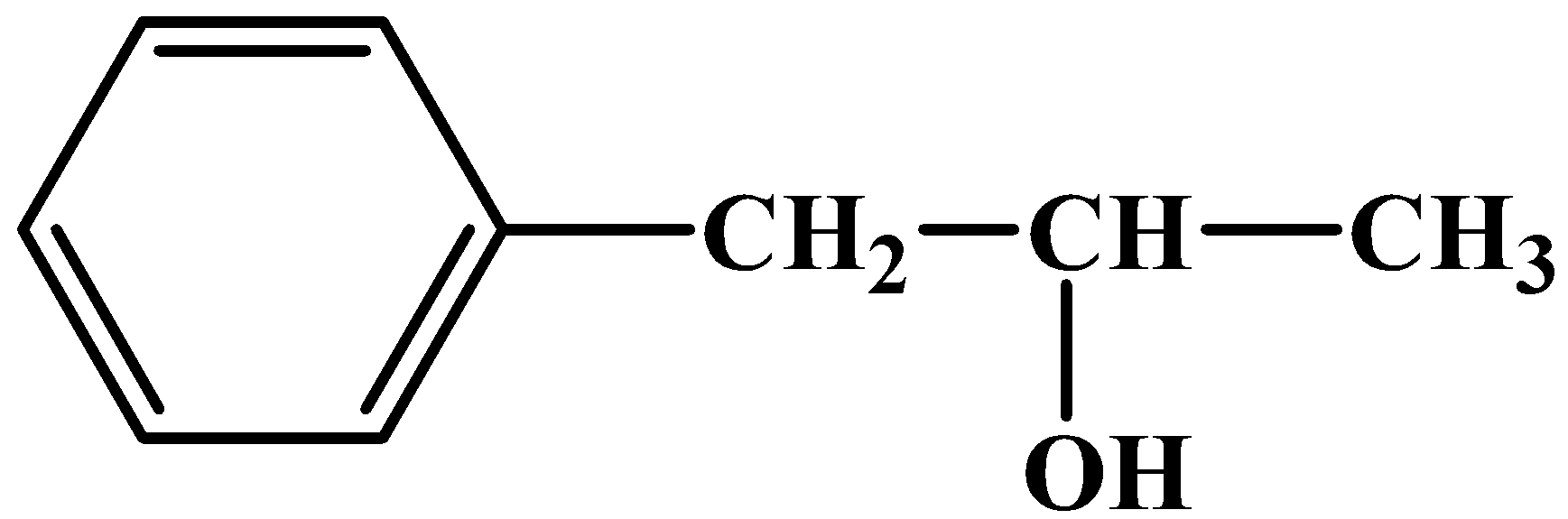
Answer
594.6k+ views
Hint: The phenyl group will be treated as a substituent while naming the given compound. The first priority functional group here is the benzene ring.
Complete answer:
Let us first try to understand what IUPAC nomenclature really refers to before applying its rules to try and name its compounds.
In general, the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. The suffix of the name reflects the type(s) of the functional group(s) present on (or within) the parent chain. Other groups which are attached to the parent chain are called substituents.
Here is a simple list of rules to follow:
- Identify the longest carbon chain. This chain is called the parent chain.
- Identify all of the substituents (groups appending from the parent chain).
- Number the carbons of the parent chain from the end that gives the substituents the lowest numbers. When comparing a series of numbers, the series that is the "lowest" is the one which contains the smallest number at the occasion of the first difference. If two or more side chains are in equivalent positions, assign the lowest number to the one which will come first in the name.
- If the same substituent occurs more than once, the location of each point on which the substituent occurs is given. In addition, the number of times the substituent group occurs is indicated by a prefix (di, tri, tetra, etc.).
- If there are two or more different substituents they are listed in alphabetical order using the base name (ignore the prefixes). The only prefix which is used when putting the substituents in alphabetical order is iso as in isopropyl or isobutyl. The prefixes sec- and tert- are not used in determining alphabetical order except when compared with each other.
- If chains of equal length are competing for selection as the parent chain, then the choice goes in series to:
a) the chain which has the greatest number of side chains.
b) the chain whose substituents have the lowest- numbers.
c) the chain having the greatest number of carbon atoms in the smaller side chain.
d)the chain having the least branched side chains.
Applying all the given rules to the given compound, we find:
- Longest Carbon chain has three carbons with no unsaturated bonds ($-propan-$).
- One aromatic substituent present on the first Carbon of the Carbon chain ($1-Phenyl-$).
- Functional group alcohol present on the second Carbon of the Carbon Chain ($-2-ol$).
Therefore, the final name of the given compound is$1-Phenylpropan-2-ol$.
Hence the answer this question is option (a).
Note: It is not necessary that the largest carbon structure will be the parent carbon. As in the above case, the benzene ring was not treated as the parent carbon although it had three more carbons than the propane group. But the phenyl group got the first position in the substituents category followed by the alcohol group. These small things should be clear to any student who wishes to write the names of these structures correctly.
Complete answer:
Let us first try to understand what IUPAC nomenclature really refers to before applying its rules to try and name its compounds.
In general, the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. The suffix of the name reflects the type(s) of the functional group(s) present on (or within) the parent chain. Other groups which are attached to the parent chain are called substituents.
Here is a simple list of rules to follow:
- Identify the longest carbon chain. This chain is called the parent chain.
- Identify all of the substituents (groups appending from the parent chain).
- Number the carbons of the parent chain from the end that gives the substituents the lowest numbers. When comparing a series of numbers, the series that is the "lowest" is the one which contains the smallest number at the occasion of the first difference. If two or more side chains are in equivalent positions, assign the lowest number to the one which will come first in the name.
- If the same substituent occurs more than once, the location of each point on which the substituent occurs is given. In addition, the number of times the substituent group occurs is indicated by a prefix (di, tri, tetra, etc.).
- If there are two or more different substituents they are listed in alphabetical order using the base name (ignore the prefixes). The only prefix which is used when putting the substituents in alphabetical order is iso as in isopropyl or isobutyl. The prefixes sec- and tert- are not used in determining alphabetical order except when compared with each other.
- If chains of equal length are competing for selection as the parent chain, then the choice goes in series to:
a) the chain which has the greatest number of side chains.
b) the chain whose substituents have the lowest- numbers.
c) the chain having the greatest number of carbon atoms in the smaller side chain.
d)the chain having the least branched side chains.
Applying all the given rules to the given compound, we find:
- Longest Carbon chain has three carbons with no unsaturated bonds ($-propan-$).
- One aromatic substituent present on the first Carbon of the Carbon chain ($1-Phenyl-$).
- Functional group alcohol present on the second Carbon of the Carbon Chain ($-2-ol$).
Therefore, the final name of the given compound is$1-Phenylpropan-2-ol$.
Hence the answer this question is option (a).
Note: It is not necessary that the largest carbon structure will be the parent carbon. As in the above case, the benzene ring was not treated as the parent carbon although it had three more carbons than the propane group. But the phenyl group got the first position in the substituents category followed by the alcohol group. These small things should be clear to any student who wishes to write the names of these structures correctly.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




