
IUPAC name of the following compound is:
$C{{H}_{3}}C{{({{C}_{2}}{{H}_{5}})}_{2}}C{{H}_{2}}Br$
Answer
532.2k+ views
Hint: Before naming the compound, we should first know about the IUPAC. The concept of IUPAC was introduced so that there is a standardized manner to name the compound all over the world and it will be the same. The motive was to give identity to every compound and a unique name.
Complete answer:
Rule for the IUPAC Nomenclature-
We have to find the parent hydrocarbon chain: It should have the maximum number of branches, substituents, length, number of multiple bonds, or single bonds.
We have to identify the parent functional group, we should know which group holds the highest priority.
Then identify the side chains that are branched from the parent chain.
Identify the functional groups that are left and see the positions they are attached to. Write those numbers as the prefix while naming.
Identify the bonds (double/triple)
Then, we have to do the numbering of the chain in the order of priority.
If a type of substituent is present more than once, then we add a prefix like di for 2, tri for 3, tetra for 4, etc.
Now, we have to find out the IUPAC name of $C{{H}_{3}}C{{({{C}_{2}}{{H}_{5}})}_{2}}C{{H}_{2}}Br$
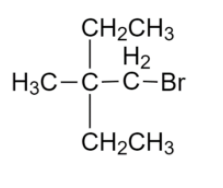
We will identify the parent hydrocarbon chain
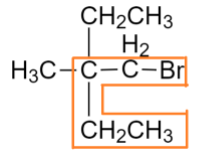
Then we have to find the parent functional group that is Bromine
We will number the chain according to the priority
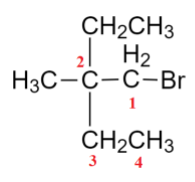
We will see the functional groups that are left that are methyl and ethyl. We can see the position of these groups, write them.
So, The IUPAC Nomenclature of this compound is 1-Bromo-2-ethyl 2-MethylButane.
Note:
Different IUPAC Methods are:
Compositional Nomenclature- The nomenclature involves naming based on the composition of the substance.
Substitutive Nomenclature- The nomenclature in which hydrogen atom gets replaced by a substituent group and the parent hydride gets changed.
Additive Nomenclature- The nomenclature is used to name the coordination compounds.
Complete answer:
Rule for the IUPAC Nomenclature-
We have to find the parent hydrocarbon chain: It should have the maximum number of branches, substituents, length, number of multiple bonds, or single bonds.
We have to identify the parent functional group, we should know which group holds the highest priority.
Then identify the side chains that are branched from the parent chain.
Identify the functional groups that are left and see the positions they are attached to. Write those numbers as the prefix while naming.
Identify the bonds (double/triple)
Then, we have to do the numbering of the chain in the order of priority.
If a type of substituent is present more than once, then we add a prefix like di for 2, tri for 3, tetra for 4, etc.
Now, we have to find out the IUPAC name of $C{{H}_{3}}C{{({{C}_{2}}{{H}_{5}})}_{2}}C{{H}_{2}}Br$

We will identify the parent hydrocarbon chain

Then we have to find the parent functional group that is Bromine
We will number the chain according to the priority

We will see the functional groups that are left that are methyl and ethyl. We can see the position of these groups, write them.
So, The IUPAC Nomenclature of this compound is 1-Bromo-2-ethyl 2-MethylButane.
Note:
Different IUPAC Methods are:
Compositional Nomenclature- The nomenclature involves naming based on the composition of the substance.
Substitutive Nomenclature- The nomenclature in which hydrogen atom gets replaced by a substituent group and the parent hydride gets changed.
Additive Nomenclature- The nomenclature is used to name the coordination compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




