
IUPAC name of methyl cyanide is:
A. Cyano nitrile
B. Ethanenitrile
C. Methane nitrile
D. Methyl-n-butyl amine
Answer
565.8k+ views
Hint: We know that naming of cyanides can be done in two ways, general naming and IUPAC naming. In the general naming, the name is in the format of the alkyl group of the compound followed by the word ‘cyanide’, such as, methyl cyanide. In methyl cyanide, a methyl group is bonded to the Cyanide group.
Complete step by step answer:
Let’s discuss the IUPAC naming of cyanides. In this type of naming, first we have to name the parent alkane. We also have to consider the carbon atom of the cyanide group in the parent alkene. The name of the parent alkane is to be followed by the word ‘nitrile’.
Now, come to the question. We have to find the correct IUPAC naming of methyl cyanide.
Let’s draw the structure of methyl cyanide.
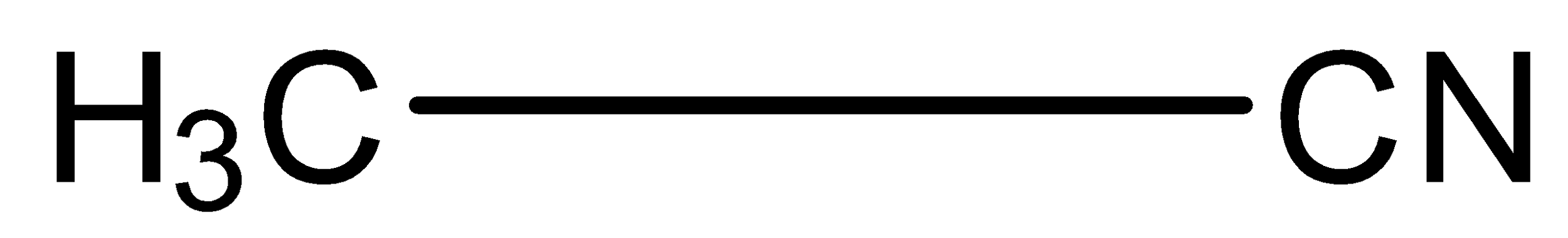
As the parent alkane contains two carbon atoms, so the name of parent alkane is ethane. And we know in the IUPAC naming of cyanide, we have to add the word ‘nitrile’ after the name of the parent chain. So, the IUPAC name of the methyl cyanide is ethanenitrile.
Hence the correct option is B.
Note: IUPAC naming is the method of naming organic compounds following a set of guidelines recommended by the international Union of Pure and Applied chemistry. The general format of naming of any organic compound is prefix + word root + suffix. The word root indicates the name of the parent chain, suffix indicates the functional group present in the compound and the prefix indicates the substituents of the compound.
Complete step by step answer:
Let’s discuss the IUPAC naming of cyanides. In this type of naming, first we have to name the parent alkane. We also have to consider the carbon atom of the cyanide group in the parent alkene. The name of the parent alkane is to be followed by the word ‘nitrile’.
Now, come to the question. We have to find the correct IUPAC naming of methyl cyanide.
Let’s draw the structure of methyl cyanide.
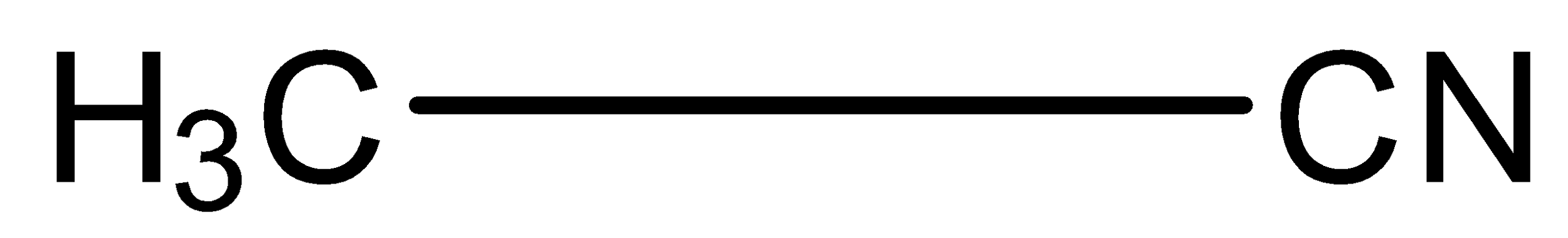
As the parent alkane contains two carbon atoms, so the name of parent alkane is ethane. And we know in the IUPAC naming of cyanide, we have to add the word ‘nitrile’ after the name of the parent chain. So, the IUPAC name of the methyl cyanide is ethanenitrile.
Hence the correct option is B.
Note: IUPAC naming is the method of naming organic compounds following a set of guidelines recommended by the international Union of Pure and Applied chemistry. The general format of naming of any organic compound is prefix + word root + suffix. The word root indicates the name of the parent chain, suffix indicates the functional group present in the compound and the prefix indicates the substituents of the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




