
What is the IUPAC name of isobutylamine:
A.Isopropyl methyl amine
B.Isopropyl methanamine
C.2-methyl-1-propanamine
D.2-methyl-3-propanamine
Answer
595.2k+ views
Hint: In IUPAC nomenclature of organic compounds, an iso-group contains one methyl group attached to the second last carbon of the continuous chain.
Complete step by step answer:
For IUPAC nomenclature of alkanes, the longest continuous carbon chain is taken as the parent chain and then it is numbered in such a way that the numbering starts from the end which is nearest to the substituent. If a functional group is present, then the longest continuous chain having the functional group is considered as the parent chain and numbering is done such that the functional group gets the lowest number.
Let us first consider the option (A) isopropyl methyl amine. It is also called N-methyl isopropyl amine. Let us draw and check its structure. It has an isopropyl group and so a methyl group will be attached to the second last carbon of the continuous chain. Also, it has a methyl amine group. So its structure will be as shown below:
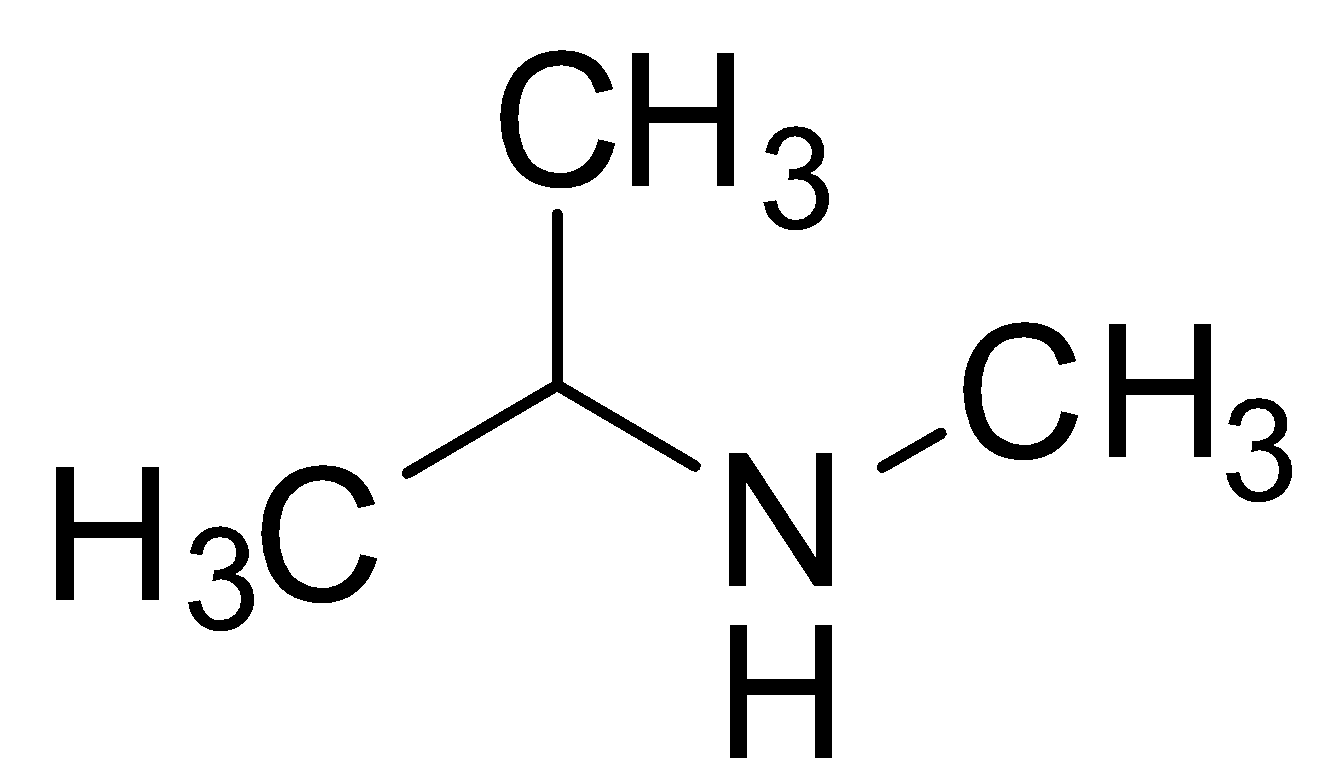
So, the option (A) is not correct.
Now, let us consider the second option which is isopropyl methanamine. It is just another name for isopropyl methyl amine. So it also has the same structure as shown above. So, the option (B) is also not correct.
Now, let us consider the third option which is 2-methyl-1-propanamine. It has the functional group amine and has a methyl group at 2- position of the continuous carbon chain. Moreover, it is a propane chain. So, its structure will be as shown below:
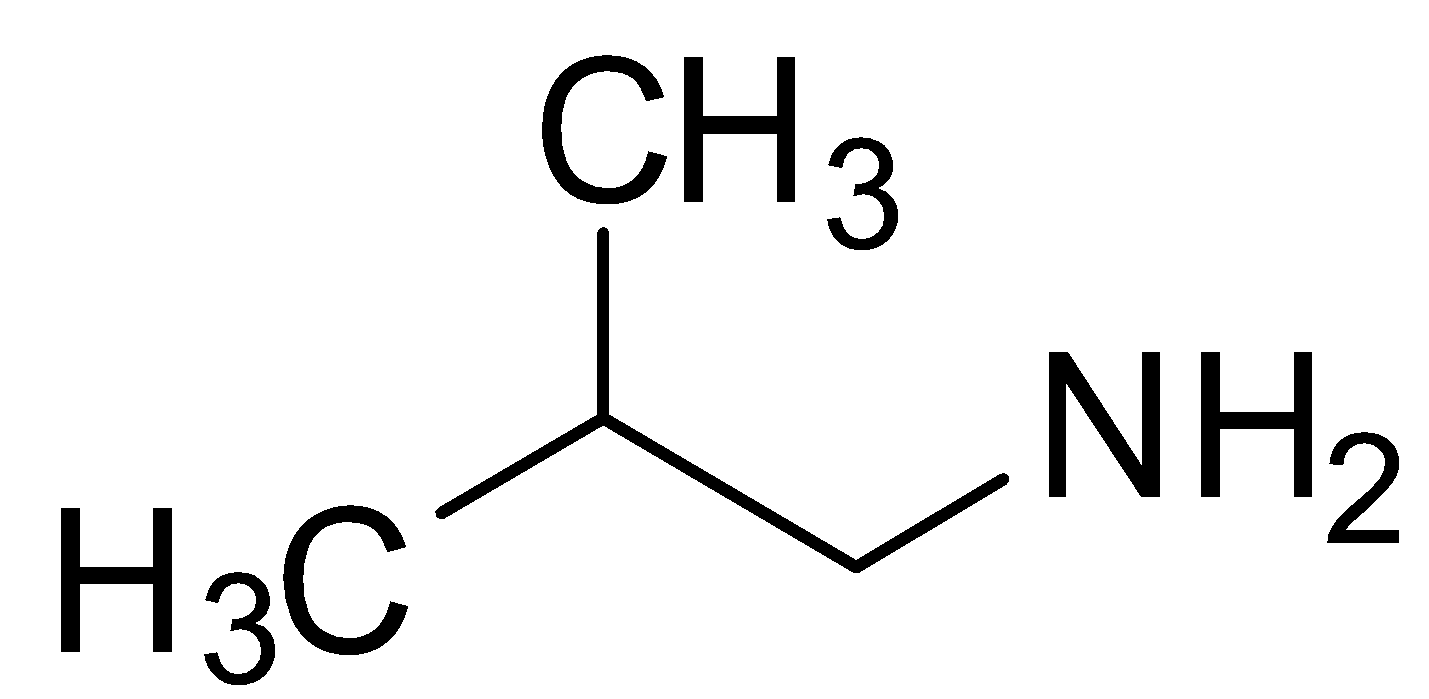
It can be observed that there is a methyl group present at the second last carbon of the propane chain containing the amine functional group. So the prefix iso-butyl can be applied while writing the common name of 2-methyl-1-propanamine. Hence, 2-methyl-1-propanamine is the IUPAC name of isobutylamine. So, the option (C) is the correct option.
Lastly, let us check the fourth option which is 2-methyl-3-propanamine. It also has the amine functional group and has a methyl group at 2- position of the continuous carbon chain. But in this case, the functional group is not given the first priority while numbering the propane chain. So, this IUPAC name is wrong. Hence, the option (D) is also not correct.
So, the only correct option is option (C).
Note:
The prefix neo is used when there are two methyl groups attached to the second last carbon of the continuous chain.
Complete step by step answer:
For IUPAC nomenclature of alkanes, the longest continuous carbon chain is taken as the parent chain and then it is numbered in such a way that the numbering starts from the end which is nearest to the substituent. If a functional group is present, then the longest continuous chain having the functional group is considered as the parent chain and numbering is done such that the functional group gets the lowest number.
Let us first consider the option (A) isopropyl methyl amine. It is also called N-methyl isopropyl amine. Let us draw and check its structure. It has an isopropyl group and so a methyl group will be attached to the second last carbon of the continuous chain. Also, it has a methyl amine group. So its structure will be as shown below:

So, the option (A) is not correct.
Now, let us consider the second option which is isopropyl methanamine. It is just another name for isopropyl methyl amine. So it also has the same structure as shown above. So, the option (B) is also not correct.
Now, let us consider the third option which is 2-methyl-1-propanamine. It has the functional group amine and has a methyl group at 2- position of the continuous carbon chain. Moreover, it is a propane chain. So, its structure will be as shown below:

It can be observed that there is a methyl group present at the second last carbon of the propane chain containing the amine functional group. So the prefix iso-butyl can be applied while writing the common name of 2-methyl-1-propanamine. Hence, 2-methyl-1-propanamine is the IUPAC name of isobutylamine. So, the option (C) is the correct option.
Lastly, let us check the fourth option which is 2-methyl-3-propanamine. It also has the amine functional group and has a methyl group at 2- position of the continuous carbon chain. But in this case, the functional group is not given the first priority while numbering the propane chain. So, this IUPAC name is wrong. Hence, the option (D) is also not correct.
So, the only correct option is option (C).
Note:
The prefix neo is used when there are two methyl groups attached to the second last carbon of the continuous chain.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




