
IUPAC name of $C{H_3}CHClC{H_2}CHO$ is _____.
Answer
582.6k+ views
Hint: In IUPAC system, the name of an organic compound consists of three parts-
(i)Word root-which denotes the number of carbon atoms present in the chain.
(ii) Suffix- Which will indicate the nature of linkage in the carbon atom like whether the carbon-carbon atoms are linked with single bond, double bond or triple bond. It also indicates the presence of a functional group.
(iii) Prefix- which represents the side chain or groups which are not regarded as functional groups.
Complete step by step answer:
Given compound is $C{H_3}CHClC{H_2}CHO$. We have to give its IUPAC name.
First we will select the longest chain containing the carbon atom having a functional group. This is the parent chain.
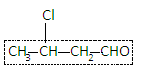
Then we will give numbers to the atoms in the parent chain such that the carbon bearing the functional group gets the lowest number. So we will number the carbon of the CHO group as $1$.
Here the chain contains four carbons and carbons are attached to each other with a single bond so the compound should be ${\text{butanal}}$.
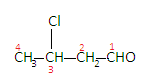
Here chlorine is present as a substituent in addition to the aldehydes group so it will be named as prefix and the number of the carbon with which it is attached will also be indicated, so the name becomes-\[{\text{2 - chlorobutanal}}\].
The IUPAC name of $C{H_3}CHClC{H_2}CHO$ is\[{\text {2 - chlorobutanal}}\].
Note:
The students may go wrong if they do not count the carbon of the aldehyde group in the parent chain. Because they are counted in the parent chain and are attached at the end of the chain. Aldehydes are also called alkanals in the IUPAC system.
(i)Word root-which denotes the number of carbon atoms present in the chain.
(ii) Suffix- Which will indicate the nature of linkage in the carbon atom like whether the carbon-carbon atoms are linked with single bond, double bond or triple bond. It also indicates the presence of a functional group.
(iii) Prefix- which represents the side chain or groups which are not regarded as functional groups.
Complete step by step answer:
Given compound is $C{H_3}CHClC{H_2}CHO$. We have to give its IUPAC name.
First we will select the longest chain containing the carbon atom having a functional group. This is the parent chain.
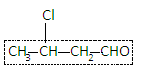
Then we will give numbers to the atoms in the parent chain such that the carbon bearing the functional group gets the lowest number. So we will number the carbon of the CHO group as $1$.
Here the chain contains four carbons and carbons are attached to each other with a single bond so the compound should be ${\text{butanal}}$.
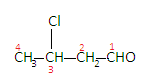
Here chlorine is present as a substituent in addition to the aldehydes group so it will be named as prefix and the number of the carbon with which it is attached will also be indicated, so the name becomes-\[{\text{2 - chlorobutanal}}\].
The IUPAC name of $C{H_3}CHClC{H_2}CHO$ is\[{\text {2 - chlorobutanal}}\].
Note:
The students may go wrong if they do not count the carbon of the aldehyde group in the parent chain. Because they are counted in the parent chain and are attached at the end of the chain. Aldehydes are also called alkanals in the IUPAC system.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




