
IUPAC name of $C{H_3} - CH = CH - C{H_3}$
(A) Unsymmetrical dimethyl ethylene
(B) Symmetrical dimethyl ethylene
(C) 1,2- dimethylethene
(D) 2-butene
Answer
569.7k+ views
Hint: We can see that the compound is symmetrical about the double bond. This means that there will be no problem in numbering the carbon atoms as well as in choosing the main carbon chain. After identifying the root word, write the suffix for alkene in accordance to the IUPAC conventions and nomenclature.
Complete Solution :
There are few rules to be followed to name a compound. Let us see those.
-Find the parent hydrocarbon.
Firstly, we have to choose the longest chain. If two different chains of equal length are present, choose the one which has the most substituents. Here there is only one chain in the compound.
-Count the number of carbon atoms present in the chain. IUPAC have fixed some prefixes based on the number of carbon atoms.
Example for 1 carbon atom, the prefix is “meth”. For 2 carbon – “eth”, “prop” for 3, “but” for 4, “pent” for 5 and so on.
There are 4 carbon atoms in $C{H_3} - CH = CH - C{H_3}$. So, the prefix is “But”.
- See if there is any double or triple bond, functional group, substituent in the main chain. Then start counting from that side from which it comes on the lowest number.
In this case, there is a double bond between the carbon atoms 2 and 3. So the suffix is “ene”.
-Name the compound. The name of the compound is 2-butene.
So, the correct answer is “Option D”.
Note: In the above question it was easy to choose the longest carbon chain and the main functional group. However, when we have to choose between the main functional group and longest chain, we give priority to the functional group. The above explanation is shown below:
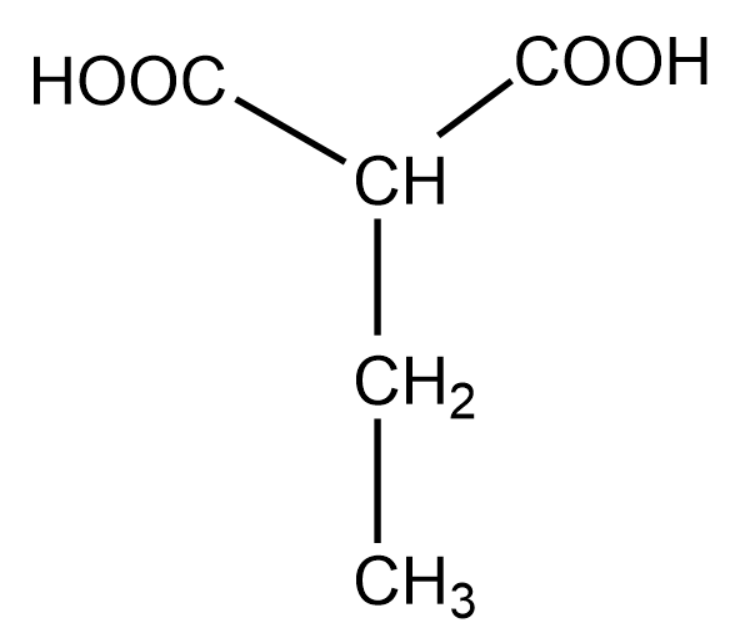
For the above organic compound, the IUPAC name is 2-ethylethan 1,3-dioic acid.
Complete Solution :
There are few rules to be followed to name a compound. Let us see those.
-Find the parent hydrocarbon.
Firstly, we have to choose the longest chain. If two different chains of equal length are present, choose the one which has the most substituents. Here there is only one chain in the compound.
-Count the number of carbon atoms present in the chain. IUPAC have fixed some prefixes based on the number of carbon atoms.
Example for 1 carbon atom, the prefix is “meth”. For 2 carbon – “eth”, “prop” for 3, “but” for 4, “pent” for 5 and so on.
There are 4 carbon atoms in $C{H_3} - CH = CH - C{H_3}$. So, the prefix is “But”.
- See if there is any double or triple bond, functional group, substituent in the main chain. Then start counting from that side from which it comes on the lowest number.
In this case, there is a double bond between the carbon atoms 2 and 3. So the suffix is “ene”.
-Name the compound. The name of the compound is 2-butene.
So, the correct answer is “Option D”.
Note: In the above question it was easy to choose the longest carbon chain and the main functional group. However, when we have to choose between the main functional group and longest chain, we give priority to the functional group. The above explanation is shown below:

For the above organic compound, the IUPAC name is 2-ethylethan 1,3-dioic acid.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




