
IUPAC name of $C{H_3} - CH = CH - CH = C = CH - CH = C = C = CH - C{H_3}$ is:
Answer
570k+ views
Hint: The above given compound contains single bonds and double bonds both in a long carbon chain. To name this compound we will use IUPAC naming system, which is a predefined naming system for hydrocarbon compounds. For a variety of compounds there are predefined IUPAC nomenclature for the naming system. The process is almost the same of every compound. The variation in the naming can be due to different functional groups present in the chain and also due to presence of higher degree of compounds as a minor branch.
Complete step by step answer:
According to the IUPAC naming system, we need to first identify the long chain of carbon.
1.Since, there are no branches or additional groups, the compound itself is a long carbon chain. Let’s number the carbon atoms.
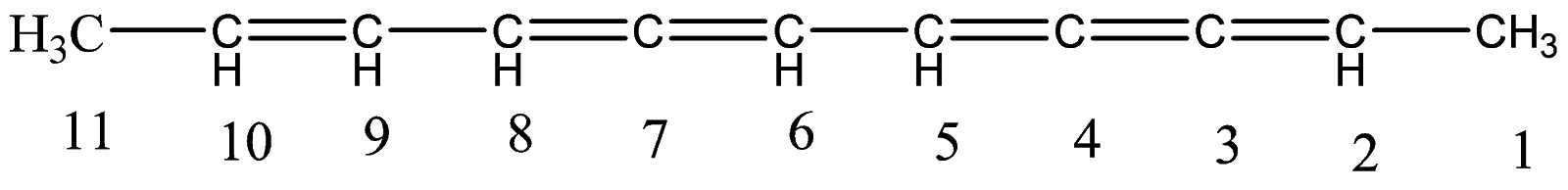
2.So here, there are a total eleven carbon atoms present in the long chain. And we have numbered each carbon atom starting from right to left as the carbon atoms at the right have more number of double bonds sequentially.
3.Hence, the naming will start with - $Undec - $, where undec means number eleven, representing the total number of carbon atoms present in the longest carbon chain.
4.Since, there are no other groups present, we will look into the bonding.
5.There are many double bonds present in the compound.
6.Let’s identify the number of double bonds present on the respective carbon atoms.
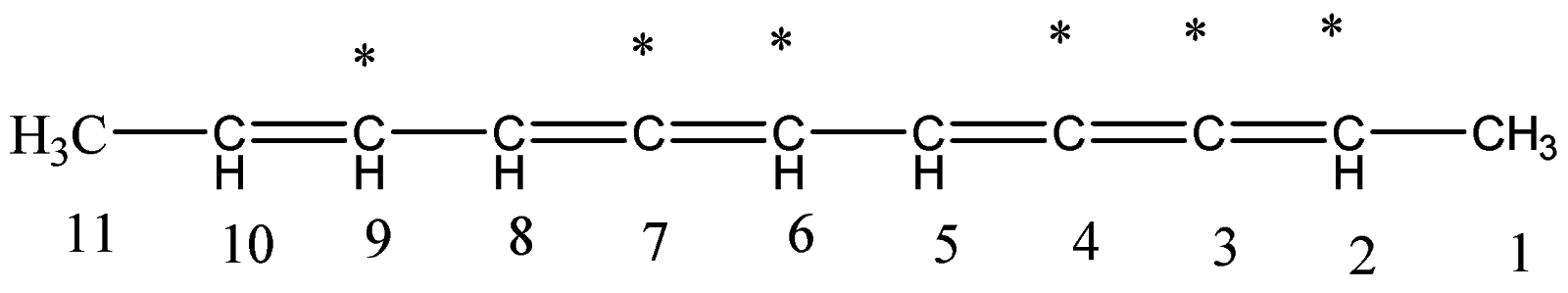
7.The double bonds are present on carbon atom number 2,3,4,6,7 and 9. There are total six such
bonds.
8.To write it in IUPAC form, first we will write the positions of the double bonds and then we will write the number of bonds and end the name by writing $ene$, since this is a double bonded compound.
$2,3,4,6,7,9 - hexene$
The overall IUPAC name of the compound will be –
$Undec - 2,3,4,6,7,9 - hexene$
Additional information:
To understand this concept more, let us take an example of an alkene compound with a functional group.
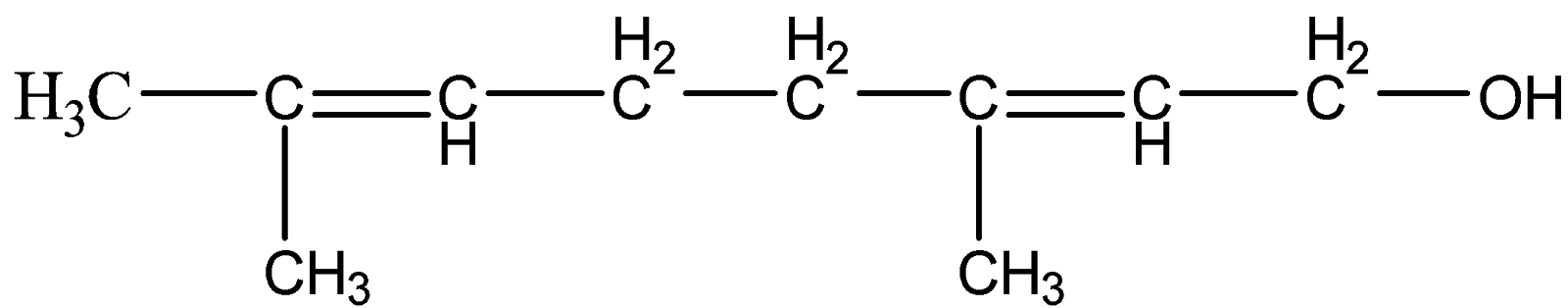 This is geraniol. Here, let us follow the IUPAC naming system.
This is geraniol. Here, let us follow the IUPAC naming system.
Since, there is one functional group present – OH. We will give priority to the functional group than double bond and figure out the longest carbon chain. Hence, the numbering will start from the carbon attached to OH group and the suffix will be -ol.
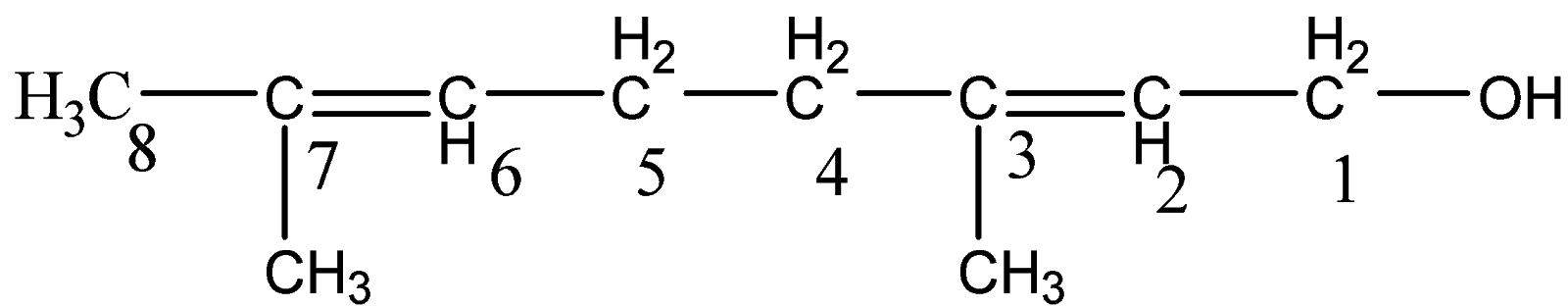
Accordingly, the longest chain is of eight carbon atoms. The name of the parent name will be octa-.
OH group is on carbon 1 and the two double bonds are on carbon 2 and carbon 6.
Hence, the name will be - $octa - 2,6 - dien - 1 - ol$.
Also we have two methyl groups attached to carbon 3 and carbon 7. There will be addition
in prefix with – $3,7 - {\text{dimethyl}}$
Hence, the overall name of the compound is written as –
$3,7 - {\text{dimethylocta}} - 2,6 - {\text{dien}} - 1 - ol$
Note: We know that all alkanes do not have linear-chain. Some alkanes are said to be in branched forms. We have to know that branched alkanes differ from straight-chain alkanes in the chains of carbon atoms which displace a few hydrogen atoms found along the chain. Substituent is the term given to groups (or) atoms that replace hydrogen in an alkane.
Complete step by step answer:
According to the IUPAC naming system, we need to first identify the long chain of carbon.
1.Since, there are no branches or additional groups, the compound itself is a long carbon chain. Let’s number the carbon atoms.

2.So here, there are a total eleven carbon atoms present in the long chain. And we have numbered each carbon atom starting from right to left as the carbon atoms at the right have more number of double bonds sequentially.
3.Hence, the naming will start with - $Undec - $, where undec means number eleven, representing the total number of carbon atoms present in the longest carbon chain.
4.Since, there are no other groups present, we will look into the bonding.
5.There are many double bonds present in the compound.
6.Let’s identify the number of double bonds present on the respective carbon atoms.

7.The double bonds are present on carbon atom number 2,3,4,6,7 and 9. There are total six such
bonds.
8.To write it in IUPAC form, first we will write the positions of the double bonds and then we will write the number of bonds and end the name by writing $ene$, since this is a double bonded compound.
$2,3,4,6,7,9 - hexene$
The overall IUPAC name of the compound will be –
$Undec - 2,3,4,6,7,9 - hexene$
Additional information:
To understand this concept more, let us take an example of an alkene compound with a functional group.

Since, there is one functional group present – OH. We will give priority to the functional group than double bond and figure out the longest carbon chain. Hence, the numbering will start from the carbon attached to OH group and the suffix will be -ol.
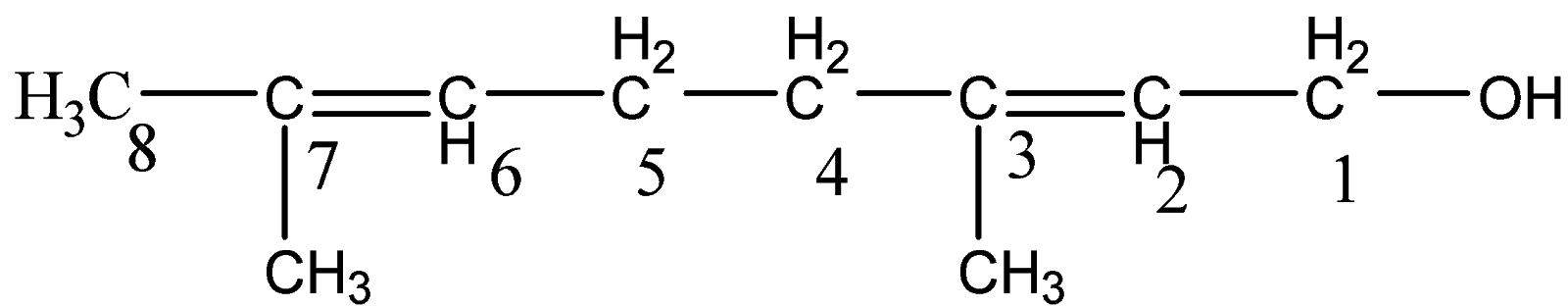
Accordingly, the longest chain is of eight carbon atoms. The name of the parent name will be octa-.
OH group is on carbon 1 and the two double bonds are on carbon 2 and carbon 6.
Hence, the name will be - $octa - 2,6 - dien - 1 - ol$.
Also we have two methyl groups attached to carbon 3 and carbon 7. There will be addition
in prefix with – $3,7 - {\text{dimethyl}}$
Hence, the overall name of the compound is written as –
$3,7 - {\text{dimethylocta}} - 2,6 - {\text{dien}} - 1 - ol$
Note: We know that all alkanes do not have linear-chain. Some alkanes are said to be in branched forms. We have to know that branched alkanes differ from straight-chain alkanes in the chains of carbon atoms which displace a few hydrogen atoms found along the chain. Substituent is the term given to groups (or) atoms that replace hydrogen in an alkane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




