
What is the isotope symbol for Iodine-131?
Answer
504k+ views
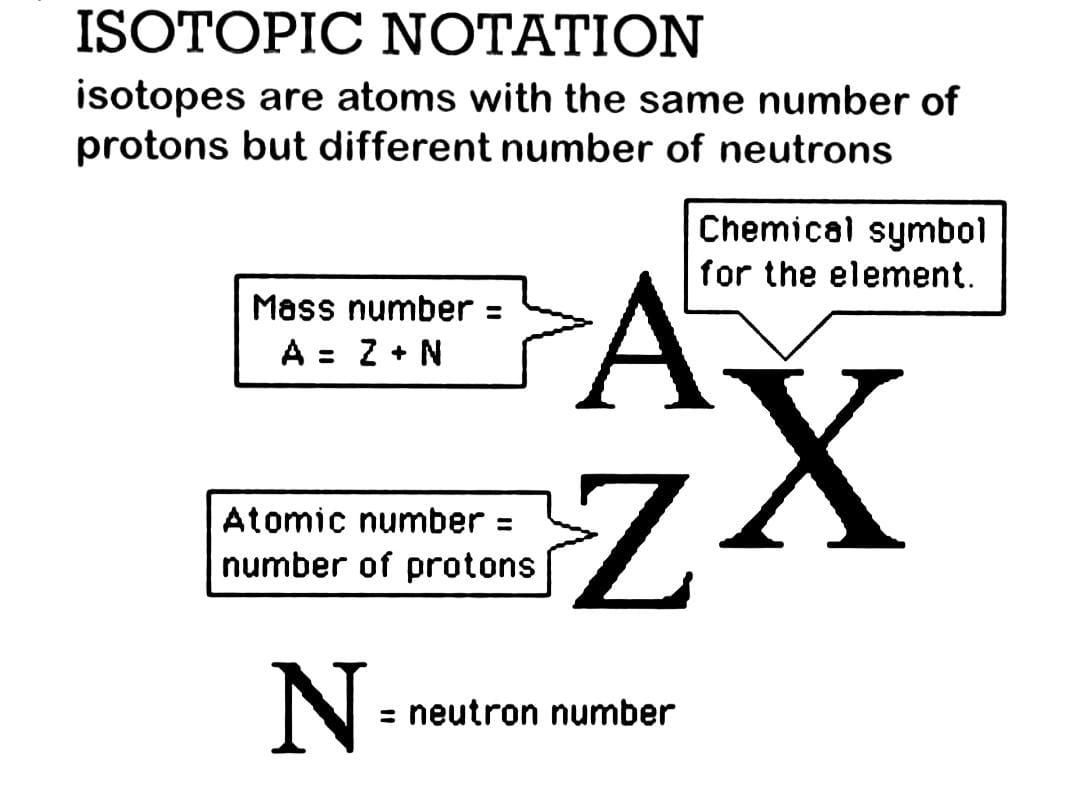
Hint :In order to answer this question, to write the symbol for an isotope, put the atomic number as a subscript to the left of the atomic symbol and the mass number (protons plus neutrons) as a superscript.
Complete Step By Step Answer:
Iodine-131 has the isotope symbol of $ {}_{53}^{131}I $
Explanation:
The isotope sign, also known as isotope notation or nuclear notation, represents an isotope's atomic number $ ,Z, $ as well as its mass number, $ A $ . On the left-hand side of the element's chemical symbol, $ X, $ the mass number is expressed as a superscript. On the left-hand side of the element's chemical symbol, the atomic number (number of protons) is expressed as a subscript.
The mass number $ \left( A \right) $ of Iodine-131 is $ 131 $ , and the atomic number $ \left( Z \right) $ is $ 53 $ . As a result, the nuclear symbol for Iodine-131 is $ {}_{53}^{131}I $ .
Additional Information:
Iodine-131 is a significant iodine radioisotope. It has an eight-day half-life in terms of radioactive decay. Nuclear energy, medical diagnostic and treatment operations, and natural gas production are all linked to it. It also plays an important function as a radioactive isotope found in nuclear fission products, and it contributed significantly to the health risks associated with open-air atomic bomb testing.
Note :
Iodine-131 is a gamma-emitting radioactive industrial tracer that is widely used. To determine the injection profile and location of fractures caused by hydraulic fracturing, radioactive tracer isotopes are injected with hydraulic fracturing fluid.
Complete Step By Step Answer:
Iodine-131 has the isotope symbol of $ {}_{53}^{131}I $
Explanation:
The isotope sign, also known as isotope notation or nuclear notation, represents an isotope's atomic number $ ,Z, $ as well as its mass number, $ A $ . On the left-hand side of the element's chemical symbol, $ X, $ the mass number is expressed as a superscript. On the left-hand side of the element's chemical symbol, the atomic number (number of protons) is expressed as a subscript.
The mass number $ \left( A \right) $ of Iodine-131 is $ 131 $ , and the atomic number $ \left( Z \right) $ is $ 53 $ . As a result, the nuclear symbol for Iodine-131 is $ {}_{53}^{131}I $ .

Additional Information:
Iodine-131 is a significant iodine radioisotope. It has an eight-day half-life in terms of radioactive decay. Nuclear energy, medical diagnostic and treatment operations, and natural gas production are all linked to it. It also plays an important function as a radioactive isotope found in nuclear fission products, and it contributed significantly to the health risks associated with open-air atomic bomb testing.
Note :
Iodine-131 is a gamma-emitting radioactive industrial tracer that is widely used. To determine the injection profile and location of fractures caused by hydraulic fracturing, radioactive tracer isotopes are injected with hydraulic fracturing fluid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




