
Isostructural species are those which have the same shape and hybridization. Among the given species, identify the isostructural pairs.
a.) \[N{{F}_{3}}\]and \[B{{F}_{3}}\]
b.) \[B{{F}_{4}}^{-}\]and \[NH_{4}^{+}\]
c.) \[BC{{l}_{3}}\]and \[BrC{{l}_{3}}\]
d.) \[N{{H}_{3}}\]and \[NO_{3}^{-}\]
Answer
595.2k+ views
Hint: For finding isostructural species we have to find hybridization of every species of central atom then we will draw the structure of given species and find the name of their structure.
Complete step by step solution:
Isostructural chemical compounds have similar chemical structures.
\[N{{F}_{3}}\]: In case of \[N{{F}_{3}}\], we know that nitrogen has five valence electrons in its outer shell. And it is making 3 bonds with fluorine, \[N{{F}_{3}}\] has one lone pair and three bond pairs. So, the hybridization is \[s{{p}^{3}}\]. And the shape is pyramidal.
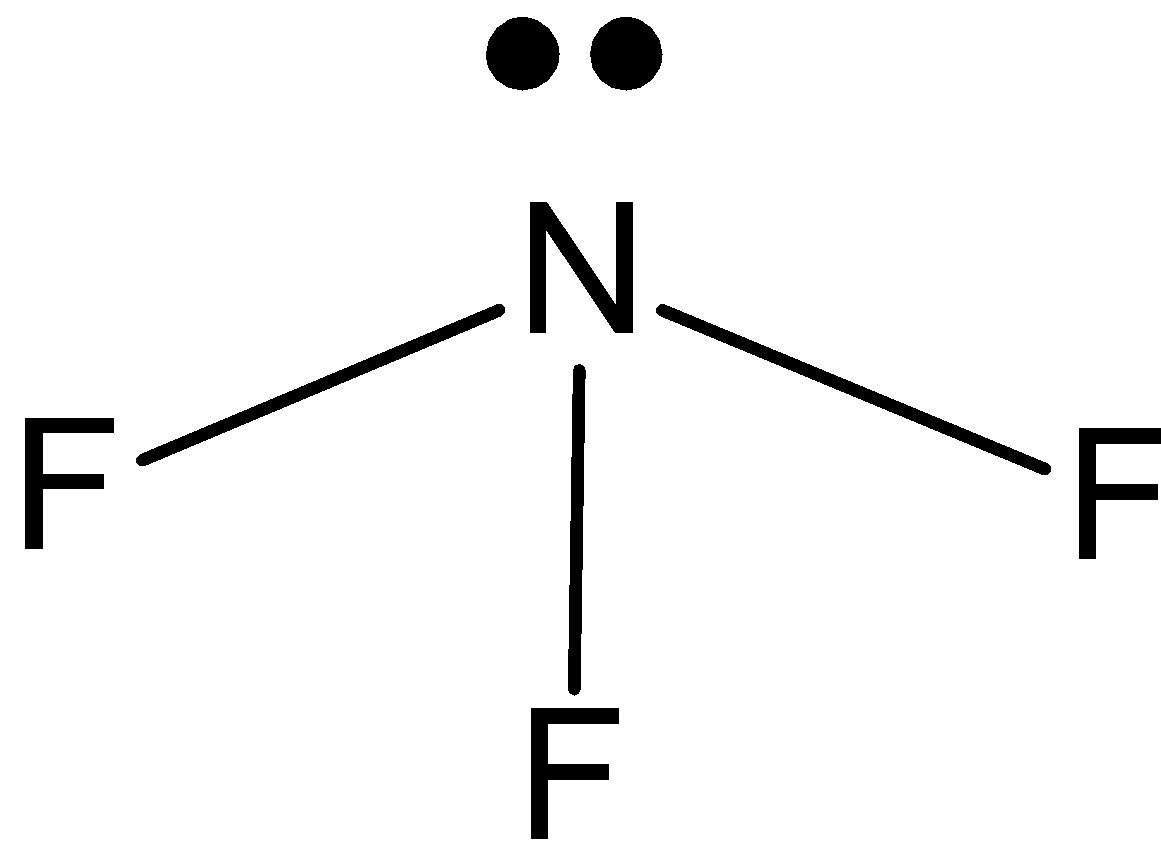
\[B{{F}_{3}}\]: In case of \[B{{F}_{3}}\], we know that boron has three valence electrons in its outer shell. And it is making 3 bonds with fluorine, \[B{{F}_{3}}\] has only three bond pairs. So, the hybridization is\[s{{p}^{2}}\]. And the shape is triangular.
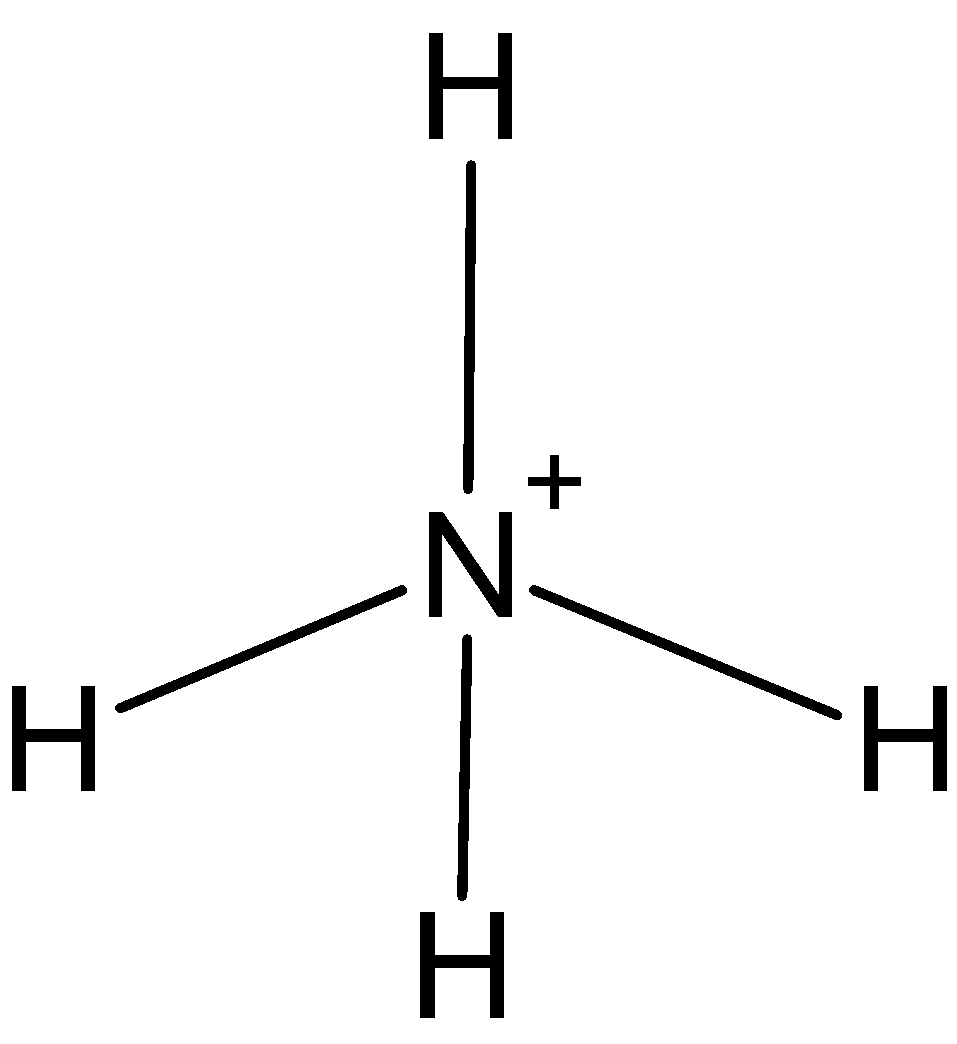
\[NH_{4}^{+}\]: In case of\[NH_{4}^{+}\], we know that nitrogen has five valence electrons in its outer shell. One positive charge is on it so it is making 4 bonds with hydrogen, \[NH_{4}^{+}\] has only four bond pairs. So, the hybridization is \[s{{p}^{3}}\]. And the shape is tetrahedral.
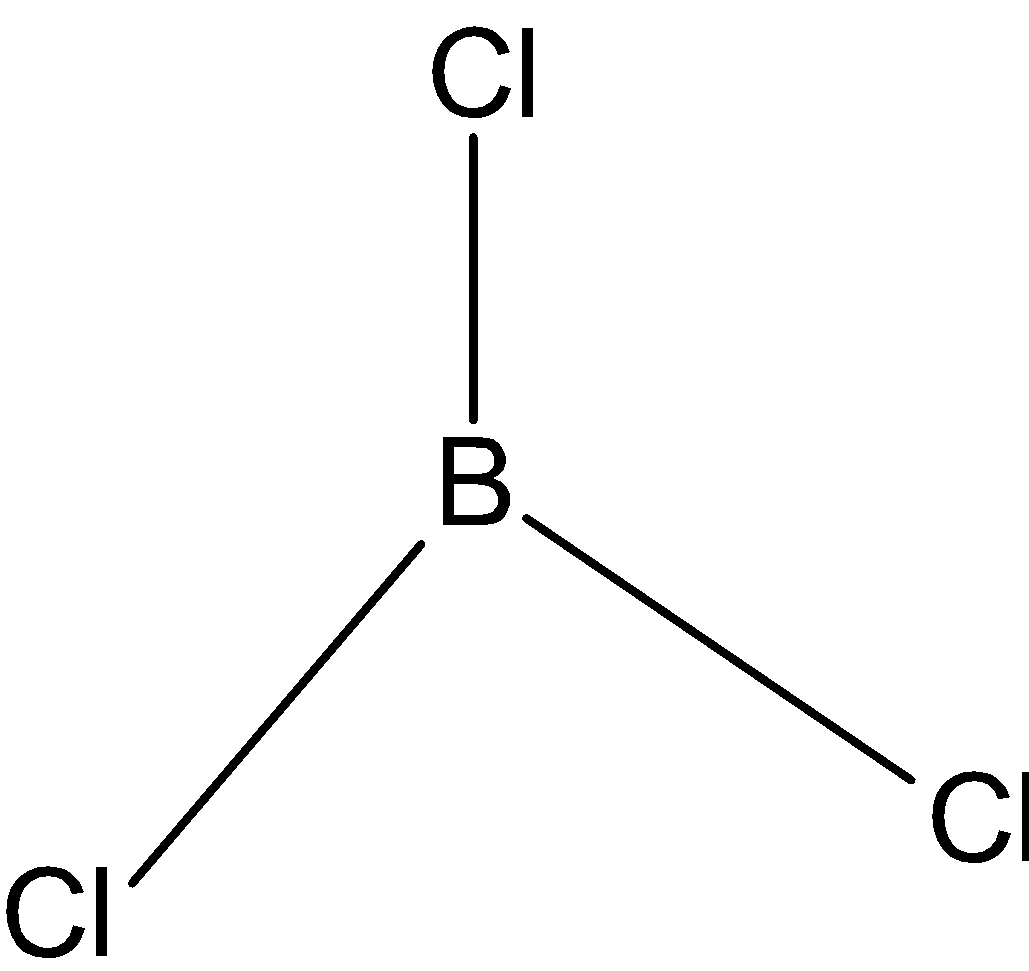
\[BC{{l}_{3}}\]: In case of \[BC{{l}_{3}}\], we know that boron has three valence electrons in its outer shell. And it is making 3 bonds with chlorine, \[BC{{l}_{3}}\] has only three bond pairs. So, the hybridization is\[s{{p}^{2}}\]. And the shape is triangular.
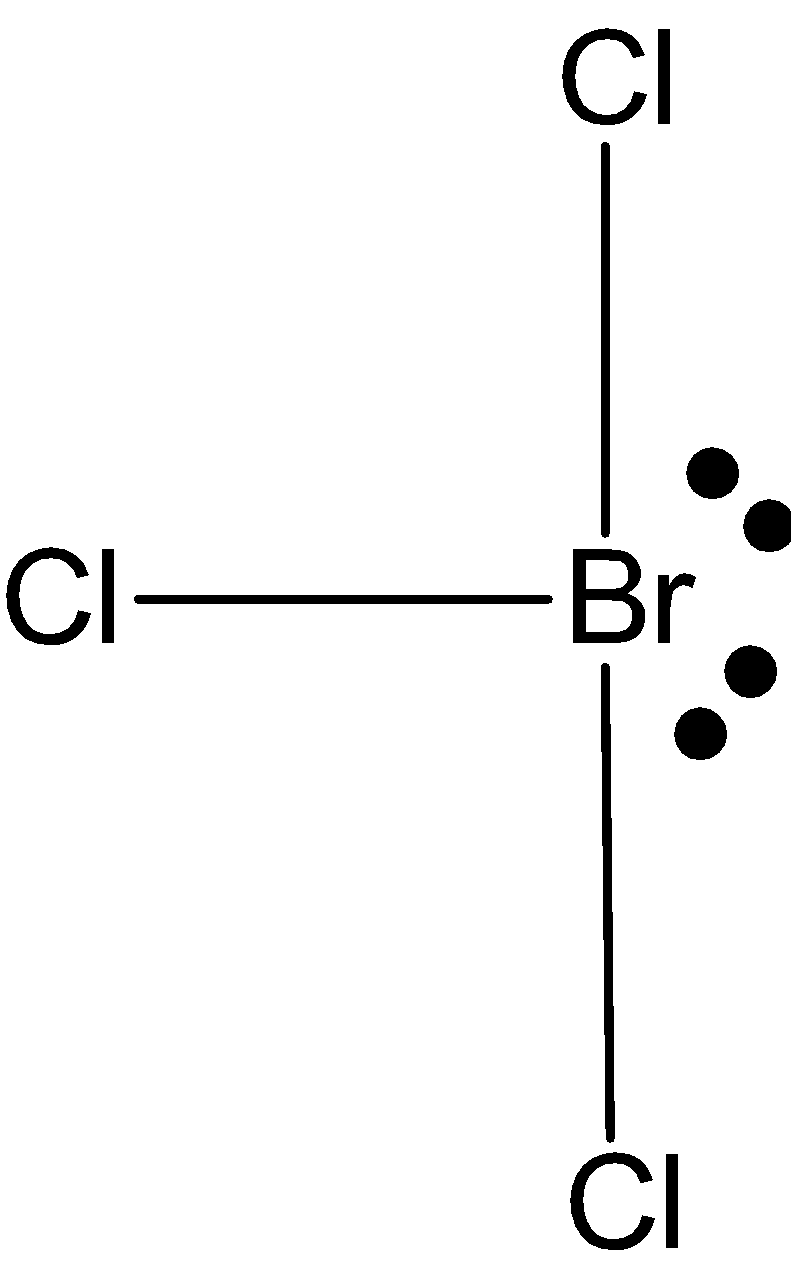
\[BrC{{l}_{3}}\]: In case of\[BrC{{l}_{3}}\], we know that bromine has seven valence electrons in its outer shell. And it is making 3 bonds with chlorine, \[BrC{{l}_{3}}\] has only three bond pairs and rest two lone pairs. So, the hybridization is\[s{{p}^{3}}d\]. But the shape is T-shape.
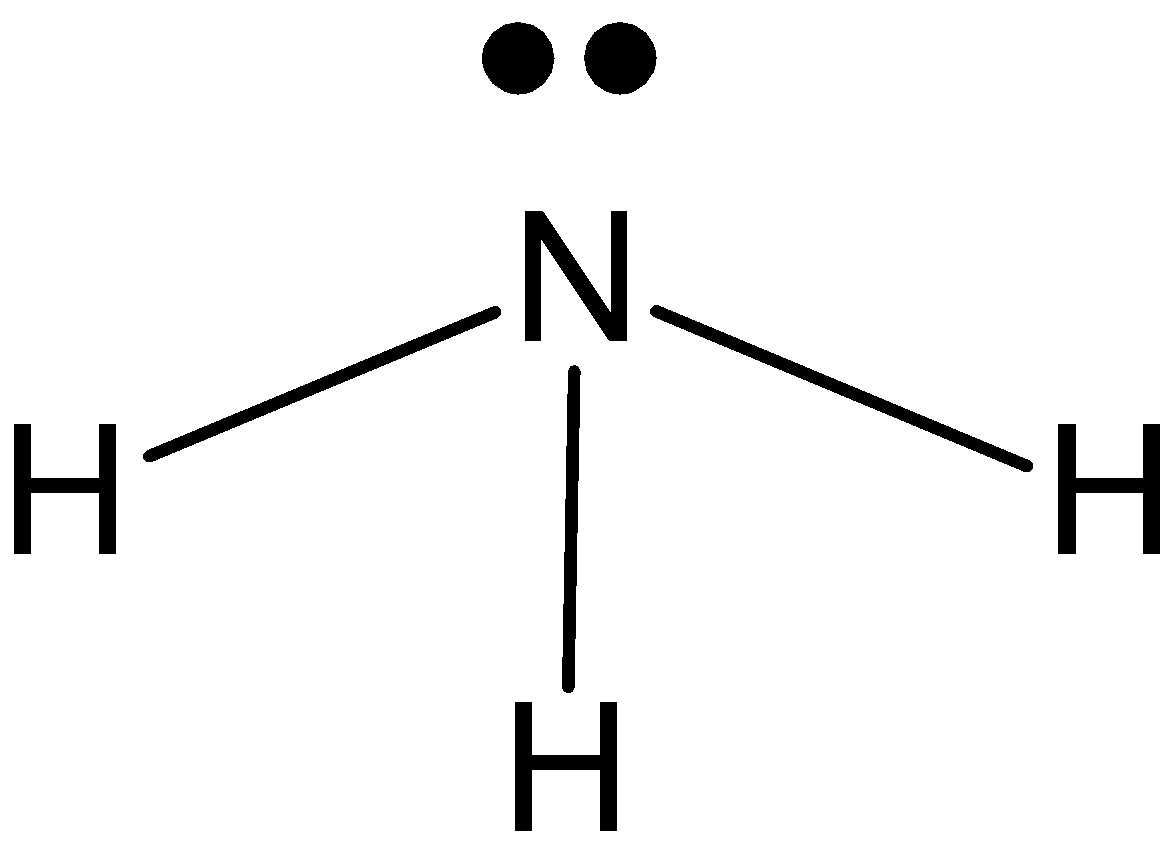
\[N{{H}_{3}}\]: In case of\[N{{H}_{3}}\], we know that nitrogen has five valence electrons in its outer shell. And it is making 3 bonds with hydrogen, \[N{{H}_{3}}\] has one lone pair and three bond pairs. So, the hybridization is\[s{{p}^{3}}\]. And the shape is pyramidal.
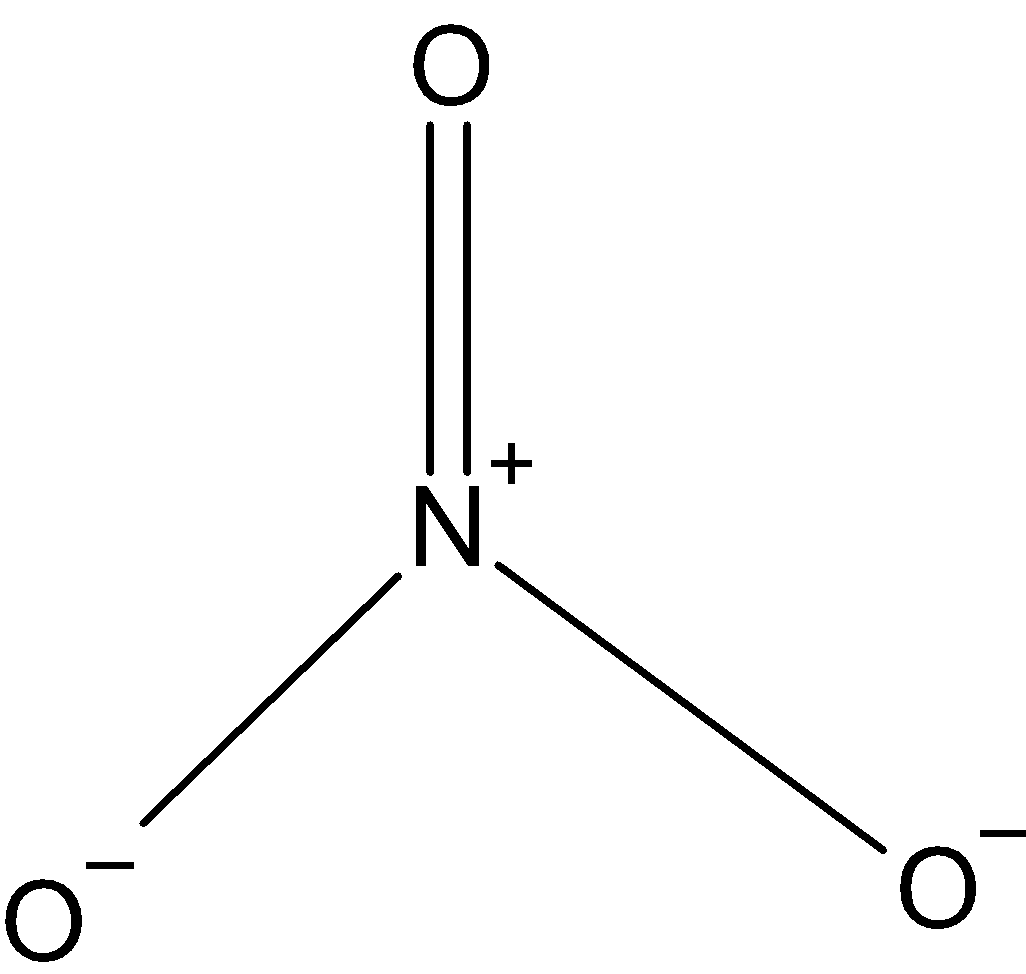
\[NO_{3}^{-}\]: In case of\[NO_{3}^{-}\], we know that nitrogen has five valence electrons in its outer shell. And it has one negative charge and it is making 4 bonds with oxygen, \[NO_{3}^{-}\] is making one double bond with single oxygen. So, the hybridization is\[s{{p}^{2}}\]. And the shape is triangular.
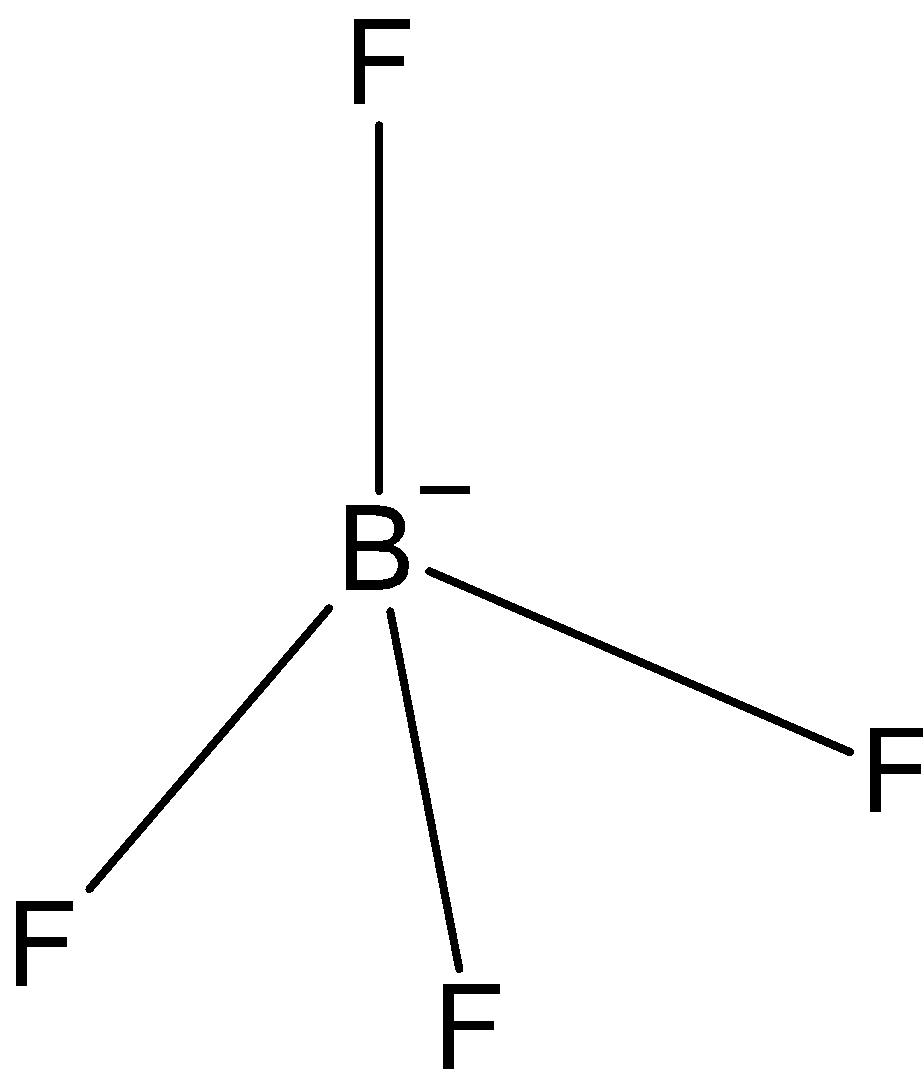
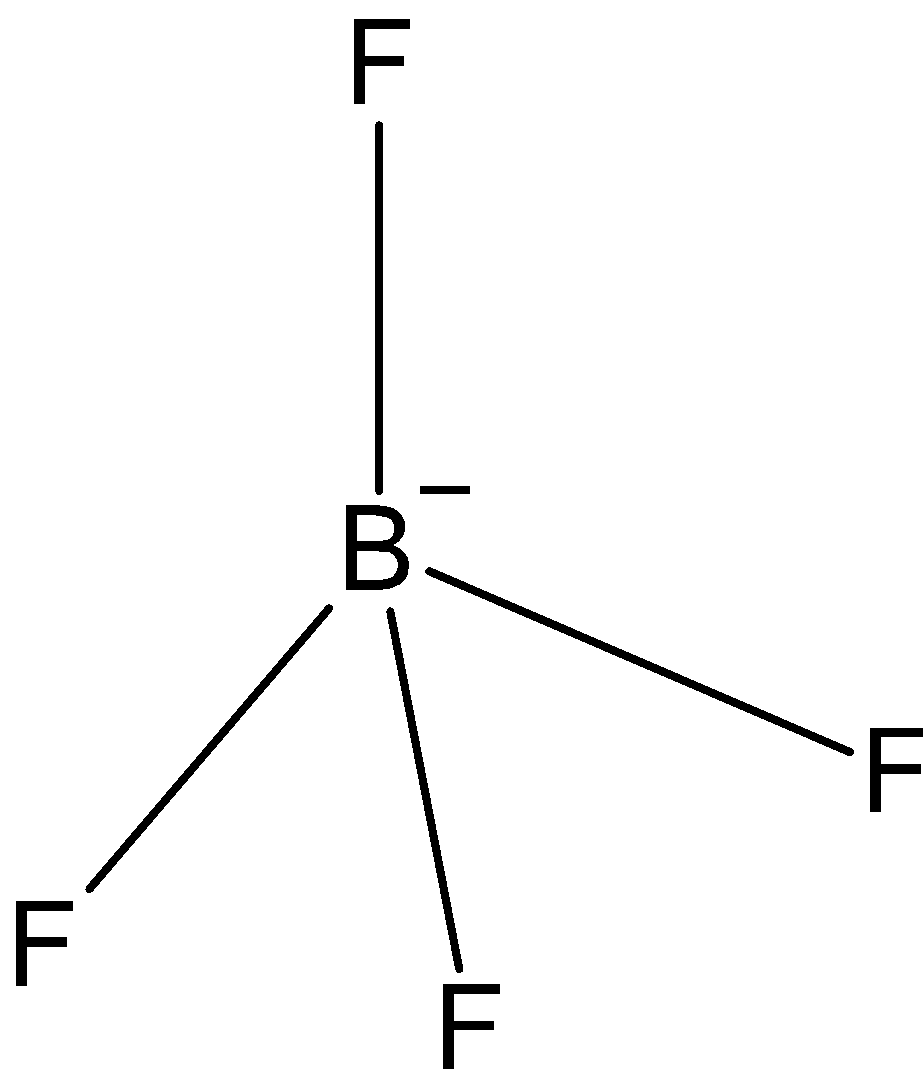
\[B{{F}_{4}}^{-}\]: In case of \[B{{F}_{4}}^{-}\], we know that boron has three valence electrons in its outer shell. And one negative charge is on it, so it is making 4 bonds with fluorine, \[B{{F}_{4}}^{-}\] has only three bond pairs. So, the hybridization is\[s{{p}^{3}}\]. And the shape is tetrahedral.
So, the correct answer is “Option B”.
Note: Here should know that in case of lone pairs we cannot predict the shape according to the hybridization, for this we have to draw the structures. And according to every hybridization you have to remember their name.
Complete step by step solution:
Isostructural chemical compounds have similar chemical structures.
\[N{{F}_{3}}\]: In case of \[N{{F}_{3}}\], we know that nitrogen has five valence electrons in its outer shell. And it is making 3 bonds with fluorine, \[N{{F}_{3}}\] has one lone pair and three bond pairs. So, the hybridization is \[s{{p}^{3}}\]. And the shape is pyramidal.

\[B{{F}_{3}}\]: In case of \[B{{F}_{3}}\], we know that boron has three valence electrons in its outer shell. And it is making 3 bonds with fluorine, \[B{{F}_{3}}\] has only three bond pairs. So, the hybridization is\[s{{p}^{2}}\]. And the shape is triangular.

\[NH_{4}^{+}\]: In case of\[NH_{4}^{+}\], we know that nitrogen has five valence electrons in its outer shell. One positive charge is on it so it is making 4 bonds with hydrogen, \[NH_{4}^{+}\] has only four bond pairs. So, the hybridization is \[s{{p}^{3}}\]. And the shape is tetrahedral.

\[BC{{l}_{3}}\]: In case of \[BC{{l}_{3}}\], we know that boron has three valence electrons in its outer shell. And it is making 3 bonds with chlorine, \[BC{{l}_{3}}\] has only three bond pairs. So, the hybridization is\[s{{p}^{2}}\]. And the shape is triangular.

\[BrC{{l}_{3}}\]: In case of\[BrC{{l}_{3}}\], we know that bromine has seven valence electrons in its outer shell. And it is making 3 bonds with chlorine, \[BrC{{l}_{3}}\] has only three bond pairs and rest two lone pairs. So, the hybridization is\[s{{p}^{3}}d\]. But the shape is T-shape.

\[N{{H}_{3}}\]: In case of\[N{{H}_{3}}\], we know that nitrogen has five valence electrons in its outer shell. And it is making 3 bonds with hydrogen, \[N{{H}_{3}}\] has one lone pair and three bond pairs. So, the hybridization is\[s{{p}^{3}}\]. And the shape is pyramidal.

\[NO_{3}^{-}\]: In case of\[NO_{3}^{-}\], we know that nitrogen has five valence electrons in its outer shell. And it has one negative charge and it is making 4 bonds with oxygen, \[NO_{3}^{-}\] is making one double bond with single oxygen. So, the hybridization is\[s{{p}^{2}}\]. And the shape is triangular.

\[B{{F}_{4}}^{-}\]: In case of \[B{{F}_{4}}^{-}\], we know that boron has three valence electrons in its outer shell. And one negative charge is on it, so it is making 4 bonds with fluorine, \[B{{F}_{4}}^{-}\] has only three bond pairs. So, the hybridization is\[s{{p}^{3}}\]. And the shape is tetrahedral.

So, the correct answer is “Option B”.
Note: Here should know that in case of lone pairs we cannot predict the shape according to the hybridization, for this we have to draw the structures. And according to every hybridization you have to remember their name.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




