
Is triethyl amine basic?
Answer
550.5k+ views
Hint: Triethyl amine can be said as the derivative of ammonia, in which the hydrogens of the ammonia is replaced with three methyl groups. The amine consists of nitrogen which has a lone pair of electrons with it.
Complete step-by-step answer:So in the question it is asked that whether the compound triethyl amine is basic or not, to come into a conclusion let us first discuss about the structure of triethylamine which may give an idea about if it is basic or not.
We know that amines are functional groups in organic chemistry which have a basic nitrogen with a lone pair and other substituents attached with them. Generally we can say that amines are the molecules which are derived from the basic molecule ammonia. The three hydrogen present in the molecule is replaced with the alkyl groups to form the alkyl amines.
There are aliphatic and aromatic amines and the triethylamine is an aliphatic amine which has three methyl groups attached with the amine functional group.
The structure of triethylamine is as follows:
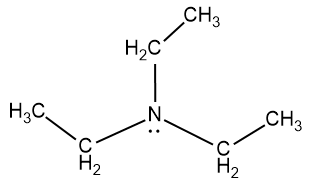
So from the structure of the molecule we know that three methyl groups are attached with the nitrogen which has a lone pair of electrons. And this lone pair of electrons in the N is available for bonding with other atoms and these electrons act as the donor atom and which makes the molecule a basic molecule.
Hence triethylamine is a basic compound. We know that the basicity of the molecule depends on the +I effect as the +I effect increases the basicity also increases. The ethyl group attached here is a +I group, hence it will be basic in nature, but compared to other primary and secondary amines it is less basic due to the steric hindrance. The three methyl groups attached makes the approach of other atoms with the lone pair of N harder making it less basic when compared with simpler alkyl amines.
Note:Triethyl amine is less basic than the ethyl amine as triethyl amine is a tertiary amine and ethyl amine is a primary amine and it is a simpler molecule in which only one ethyl group is present. Even though the +I group is less in ethyl amine but the steric hindrance factor plays no role in ethyl amine and the lone pair in the N is easily available.
Primary amines are those molecules in which only one H of ammonia is replaced with an alkyl group, in secondary amine two H atoms are replaced with two alkyl groups and in tertiary amines three alkyl groups replace the three H atoms. It also forms a fourth bond with another atom with a positive charge on the nitrogen atom.
Complete step-by-step answer:So in the question it is asked that whether the compound triethyl amine is basic or not, to come into a conclusion let us first discuss about the structure of triethylamine which may give an idea about if it is basic or not.
We know that amines are functional groups in organic chemistry which have a basic nitrogen with a lone pair and other substituents attached with them. Generally we can say that amines are the molecules which are derived from the basic molecule ammonia. The three hydrogen present in the molecule is replaced with the alkyl groups to form the alkyl amines.
There are aliphatic and aromatic amines and the triethylamine is an aliphatic amine which has three methyl groups attached with the amine functional group.
The structure of triethylamine is as follows:

So from the structure of the molecule we know that three methyl groups are attached with the nitrogen which has a lone pair of electrons. And this lone pair of electrons in the N is available for bonding with other atoms and these electrons act as the donor atom and which makes the molecule a basic molecule.
Hence triethylamine is a basic compound. We know that the basicity of the molecule depends on the +I effect as the +I effect increases the basicity also increases. The ethyl group attached here is a +I group, hence it will be basic in nature, but compared to other primary and secondary amines it is less basic due to the steric hindrance. The three methyl groups attached makes the approach of other atoms with the lone pair of N harder making it less basic when compared with simpler alkyl amines.
Note:Triethyl amine is less basic than the ethyl amine as triethyl amine is a tertiary amine and ethyl amine is a primary amine and it is a simpler molecule in which only one ethyl group is present. Even though the +I group is less in ethyl amine but the steric hindrance factor plays no role in ethyl amine and the lone pair in the N is easily available.
Primary amines are those molecules in which only one H of ammonia is replaced with an alkyl group, in secondary amine two H atoms are replaced with two alkyl groups and in tertiary amines three alkyl groups replace the three H atoms. It also forms a fourth bond with another atom with a positive charge on the nitrogen atom.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

How was the Civil Disobedience Movement different from class 12 social science CBSE

How is democracy better than other forms of government class 12 social science CBSE




