
Is toluene soluble in water?
Answer
540.3k+ views
Hint:Toluene is an aromatic compound in which a methyl group is present on the benzene ring. In water, there is strong hydrogen bonding because oxygen and hydrogen can form a hydrogen bond, so the incoming molecules must form a stronger bond than the already existing hydrogen bond.
Complete step-by-step answer:Toluene is an aromatic compound in which a methyl group is present on the benzene ring and its molecular formula is ${{C}_{7}}{{H}_{8}}$. The structure of toluene is given below:
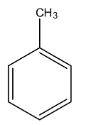
It is an aromatic hydrocarbon because there is no functional group in toluene. The molecular mass of toluene is 92.141 g /mol. Since it is an aromatic compound, its odor is sweet and benzene-like. It is a colorless liquid and has a density of 0.87 g /mL.
Its solubility in water is very less or negligible and it is equal to 0.52 g /L at ${{20}^{\circ }}C$ which means in one liter of water only 0.52 grams of toluene is soluble. This can be explained as, the water is made up of hydrogen and oxygen, and we know that oxygen is an electronegative atom so, there will be bonding between the molecules of water. Since there is no electronegative atom in toluene, the bonding formed between toluene and water is a van der Waal interaction which is a weaker interaction than the hydrogen bonding. So this weak van der Waal interaction cannot compensate for the strong already existing hydrogen bonding in the water.
Hence, toluene is not soluble in water.
Note:The boiling and melting points of toluene are 384 K and 178 K respectively. Alcohols are carboxylic acids and are quite soluble in water because both of them contain oxygen atoms for making hydrogen bonding.
Complete step-by-step answer:Toluene is an aromatic compound in which a methyl group is present on the benzene ring and its molecular formula is ${{C}_{7}}{{H}_{8}}$. The structure of toluene is given below:

It is an aromatic hydrocarbon because there is no functional group in toluene. The molecular mass of toluene is 92.141 g /mol. Since it is an aromatic compound, its odor is sweet and benzene-like. It is a colorless liquid and has a density of 0.87 g /mL.
Its solubility in water is very less or negligible and it is equal to 0.52 g /L at ${{20}^{\circ }}C$ which means in one liter of water only 0.52 grams of toluene is soluble. This can be explained as, the water is made up of hydrogen and oxygen, and we know that oxygen is an electronegative atom so, there will be bonding between the molecules of water. Since there is no electronegative atom in toluene, the bonding formed between toluene and water is a van der Waal interaction which is a weaker interaction than the hydrogen bonding. So this weak van der Waal interaction cannot compensate for the strong already existing hydrogen bonding in the water.
Hence, toluene is not soluble in water.
Note:The boiling and melting points of toluene are 384 K and 178 K respectively. Alcohols are carboxylic acids and are quite soluble in water because both of them contain oxygen atoms for making hydrogen bonding.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




