
Is methyl an activating group?
Answer
535.5k+ views
Hint: If the methyl group will direct the incoming electrophile or nucleophile to the ortho, or para positions then it will be an activating group, or if the methyl group will direct the incoming electrophile or nucleophile to the Meta position then it will be a deactivating group.
Complete answer:
Let us first understand the activating and deactivating group present on the aromatic ring. When the electrons are donated to the benzene ring, then the benzene will be activated and the rate of the reaction will be increased. If the incoming electrophile or nucleophile is attached to the ortho, or para positions then the compound will be an activating group.
When the electrons are taken from the benzene ring, then the benzene will be deactivated and the rate of the reaction will be decreased. If the incoming electrophile or nucleophile is attached to the Meta position then the compound will be a deactivating group.
When the methyl is attached to the benzene ring, then it is known as toluene. So, the incoming nucleophile will either attack the ortho or para position, or the meta position.
In toluene, the incoming compound is attached to the ortho, or para position, because the methyl group is an activating group. For example, when the toluene is treated with nitric acid, the nitro group attaches to the ortho or para position. The reaction is given below:
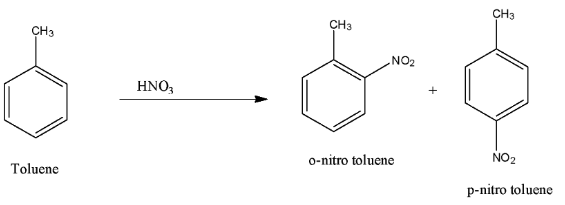
Hence, the methyl group is an activating group.
Note: But the compound on the benzene is nitro, i.e., Nitrobenzene is treated with methyl chloride, then the methyl group attaches to the meta-position because the nitro group is a deactivating group.
Complete answer:
Let us first understand the activating and deactivating group present on the aromatic ring. When the electrons are donated to the benzene ring, then the benzene will be activated and the rate of the reaction will be increased. If the incoming electrophile or nucleophile is attached to the ortho, or para positions then the compound will be an activating group.
When the electrons are taken from the benzene ring, then the benzene will be deactivated and the rate of the reaction will be decreased. If the incoming electrophile or nucleophile is attached to the Meta position then the compound will be a deactivating group.
When the methyl is attached to the benzene ring, then it is known as toluene. So, the incoming nucleophile will either attack the ortho or para position, or the meta position.
In toluene, the incoming compound is attached to the ortho, or para position, because the methyl group is an activating group. For example, when the toluene is treated with nitric acid, the nitro group attaches to the ortho or para position. The reaction is given below:

Hence, the methyl group is an activating group.
Note: But the compound on the benzene is nitro, i.e., Nitrobenzene is treated with methyl chloride, then the methyl group attaches to the meta-position because the nitro group is a deactivating group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




