
Is HCN linear, trigonal or tetrahedral?
Answer
490.2k+ views
Hint: This question will be solved purely on the basis of the geometry of the molecules that they acquire while stabilising their bonds and the way they exist in nature. To answer this question, we should have knowledge regarding the geometry of the molecules.
Complete Step By Step Answer:
This answer is very easy to answer, for this we should have knowledge regarding the geometry of the molecule.
So let’s solve this question, now coming to the molecule hydrogen cyanide $ HCN $ , so this is a linear molecule.
For a better understanding of the question, let’s have a look at the structure of the molecule hydrogen cyanide.
So, this is the molecule:
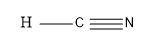
So, as we can see from the above figure that in the HCN molecule i.e. in hydrogen cyanide molecule, one electron from the hydrogen is shared with the carbon and the three electrons of the Nitrogen atom forms the triple bond with the Carbon atom and thus completing the valence of eight electrons of the carbon atom i.e. the middle atom.
So, from the above discussion , we can say that the hydrogen cyanide molecule is linear in shape and they have an angle of $ {180^o} $ between Cabin and hydrogen and carbon and nitrogen.
Note:
As given in questions, the trigonal planar symmetry consists of the four atoms , one at the middle of the equilateral triangle and three other at the corners of the triangle , the all there ligands or atoms are identical in shape and has a bond angle of $ {120^o} $ .
Complete Step By Step Answer:
This answer is very easy to answer, for this we should have knowledge regarding the geometry of the molecule.
So let’s solve this question, now coming to the molecule hydrogen cyanide $ HCN $ , so this is a linear molecule.
For a better understanding of the question, let’s have a look at the structure of the molecule hydrogen cyanide.
So, this is the molecule:
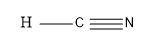
So, as we can see from the above figure that in the HCN molecule i.e. in hydrogen cyanide molecule, one electron from the hydrogen is shared with the carbon and the three electrons of the Nitrogen atom forms the triple bond with the Carbon atom and thus completing the valence of eight electrons of the carbon atom i.e. the middle atom.
So, from the above discussion , we can say that the hydrogen cyanide molecule is linear in shape and they have an angle of $ {180^o} $ between Cabin and hydrogen and carbon and nitrogen.
Note:
As given in questions, the trigonal planar symmetry consists of the four atoms , one at the middle of the equilateral triangle and three other at the corners of the triangle , the all there ligands or atoms are identical in shape and has a bond angle of $ {120^o} $ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




