
Is glycerol soluble in water?
Answer
506.7k+ views
Hint: We have to know that glycerol is a chemical compound having the chemical formula \[{C_3}{H_8}{O_3}\]. It is a simple polyol compound and it is viscous in nature. And there is no colour and odor and it is non – toxic. The glycerol is widely used in FDA due to their antiviral and antimicrobial properties. The backbone of the glycerol is found in lipids and that is known as glycerides. And glycerol is mainly used for the treatment for constipation and to improve the hydration and it also apply for some certain skin conditions.
Complete answer:
We have to remember that the glycerol is completely soluble in water because of their ability to form the hydrogen bond with water molecules by using their polyol groups, which means three hydroxyl groups are present in the glycerol. Hence, these three hydroxyl groups present in the glycerol become polarized which helps to increase its solubility in water. Therefore, the glycerol is hygroscopic in nature. Because it has the tendency to absorb water from the air. And the density of glycerol is slightly more than the water. The glycerol is also soluble in the alcohol and many of the organic solvents like dichloromethane, diethyl ether, ethyl acetate etc. But it is not soluble in the hydrocarbons. Let’s see the structure of glycerol,
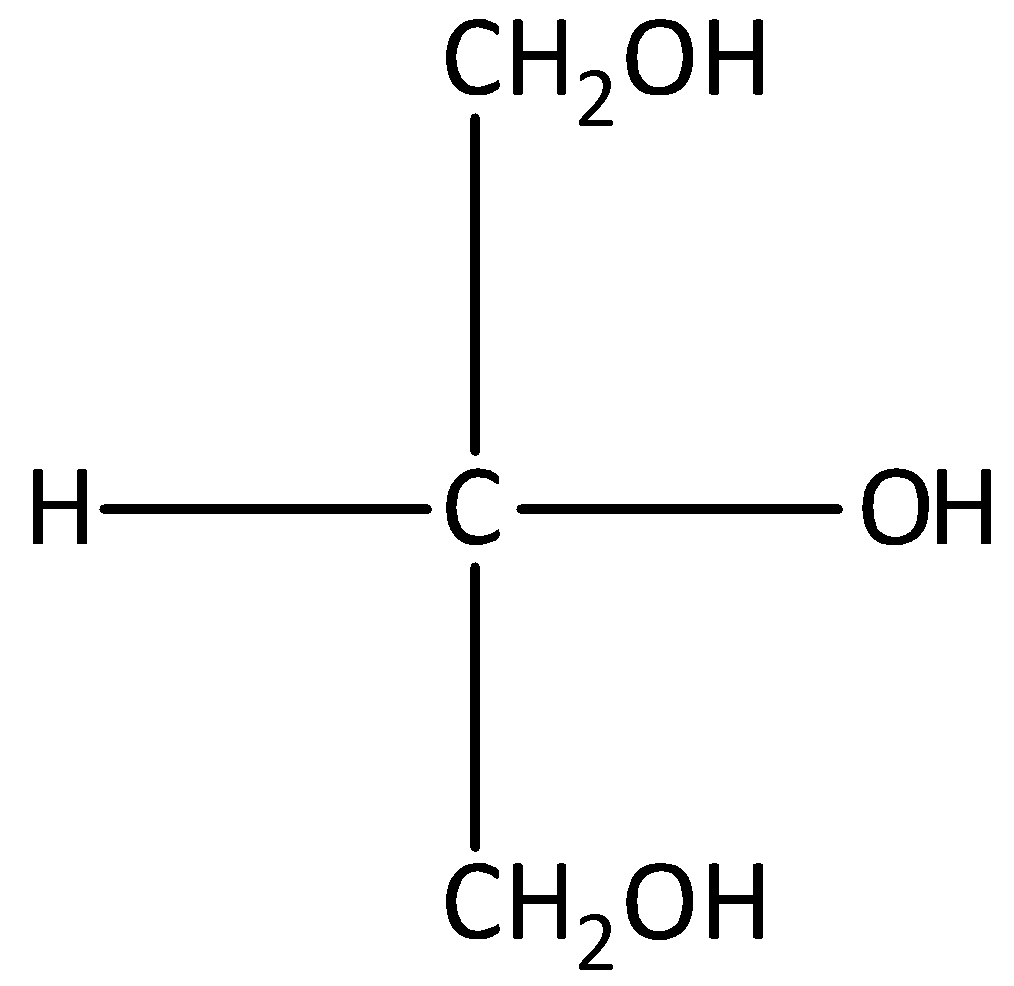
Note:
We need to know that the three hydroxyl groups are responsible for the solubility of glycerol in water. These three hydroxyl groups will be polarized. Thus, it is easy to soluble in the water. Hence, it is hygroscopic in nature, which means water loving. The glycerol is an alcohol which contains three carbon groups in their structure. And this glycerol is used for food colors and flavors as the solvent.
Complete answer:
We have to remember that the glycerol is completely soluble in water because of their ability to form the hydrogen bond with water molecules by using their polyol groups, which means three hydroxyl groups are present in the glycerol. Hence, these three hydroxyl groups present in the glycerol become polarized which helps to increase its solubility in water. Therefore, the glycerol is hygroscopic in nature. Because it has the tendency to absorb water from the air. And the density of glycerol is slightly more than the water. The glycerol is also soluble in the alcohol and many of the organic solvents like dichloromethane, diethyl ether, ethyl acetate etc. But it is not soluble in the hydrocarbons. Let’s see the structure of glycerol,

Note:
We need to know that the three hydroxyl groups are responsible for the solubility of glycerol in water. These three hydroxyl groups will be polarized. Thus, it is easy to soluble in the water. Hence, it is hygroscopic in nature, which means water loving. The glycerol is an alcohol which contains three carbon groups in their structure. And this glycerol is used for food colors and flavors as the solvent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




