
Is Ethanol an alcohol?
Answer
541.5k+ views
Hint:Alcohol is a compound which will have a functional group –OH attached with the alkyl group. In ethanol there are two carbon atoms, five H atoms and one hydroxyl group and has the molecular formula ${{C}_{2}}{{H}_{6}}O$.
Complete step-by-step answer:Before going into the solution for the question let us discuss some basic facts about alcohol.
Alcohol is a class of compounds which belongs to the organic compounds section. A alcohol molecule will have at least one hydroxyl group i.e. –OH functional group. To be more accurate we could define alcohols as the derivatives of water, as one H replaced by the alkyl group will give the alcohols. The alkyl groups can be of any number and according to the number of carbon increases different types of alcohols are formed.
If the carbon atom attached to the –OH group is connected with only one alkyl group then that class of alcohols are called as the primary alcohol, if the carbon atom is attached to more alkyl group then that class of alcohols called as secondary alcohol and the class of alcohol in which the C atom attached to –OH group is attached to more than three alkyl chain called as tertiary alcohols.
Now let us talk about ethanol. Ethanol is also called as ethyl alcohol and this ethyl alcohol is generally called as alcohol. It has a molecular formulae ${{C}_{2}}{{H}_{6}}O$ or ${{C}_{2}}{{H}_{5}}OH$.
The structure of ethanol is as follows:
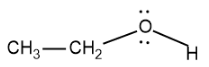
From the structure we know that the ethanol molecule has one hydroxyl group attached to an ethyl chain so we could say that ethanol is an alcohol and it is a primary alcohol.
Note:Ethanol finds application for the synthesis of anesthetic ether. It is also used for sterilizing medical purpose instruments. Ethanol is mainly used in beverages and it also finds application in making toiletries and pharmaceuticals.
Complete step-by-step answer:Before going into the solution for the question let us discuss some basic facts about alcohol.
Alcohol is a class of compounds which belongs to the organic compounds section. A alcohol molecule will have at least one hydroxyl group i.e. –OH functional group. To be more accurate we could define alcohols as the derivatives of water, as one H replaced by the alkyl group will give the alcohols. The alkyl groups can be of any number and according to the number of carbon increases different types of alcohols are formed.
If the carbon atom attached to the –OH group is connected with only one alkyl group then that class of alcohols are called as the primary alcohol, if the carbon atom is attached to more alkyl group then that class of alcohols called as secondary alcohol and the class of alcohol in which the C atom attached to –OH group is attached to more than three alkyl chain called as tertiary alcohols.
Now let us talk about ethanol. Ethanol is also called as ethyl alcohol and this ethyl alcohol is generally called as alcohol. It has a molecular formulae ${{C}_{2}}{{H}_{6}}O$ or ${{C}_{2}}{{H}_{5}}OH$.
The structure of ethanol is as follows:

From the structure we know that the ethanol molecule has one hydroxyl group attached to an ethyl chain so we could say that ethanol is an alcohol and it is a primary alcohol.
Note:Ethanol finds application for the synthesis of anesthetic ether. It is also used for sterilizing medical purpose instruments. Ethanol is mainly used in beverages and it also finds application in making toiletries and pharmaceuticals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




