
Is EDTA an Acid or Base?
Answer
502.5k+ views
Hint: EDTA stands for ethylenediaminetetraacetic acid. It is widely used in complexometric titrations and is a well-known metal-chelating agent, extensively used for the treatment of heavy metal poisoning. EDTA has four groups of carboxyl and two groups of amines.
The structure of EDTA is given below-
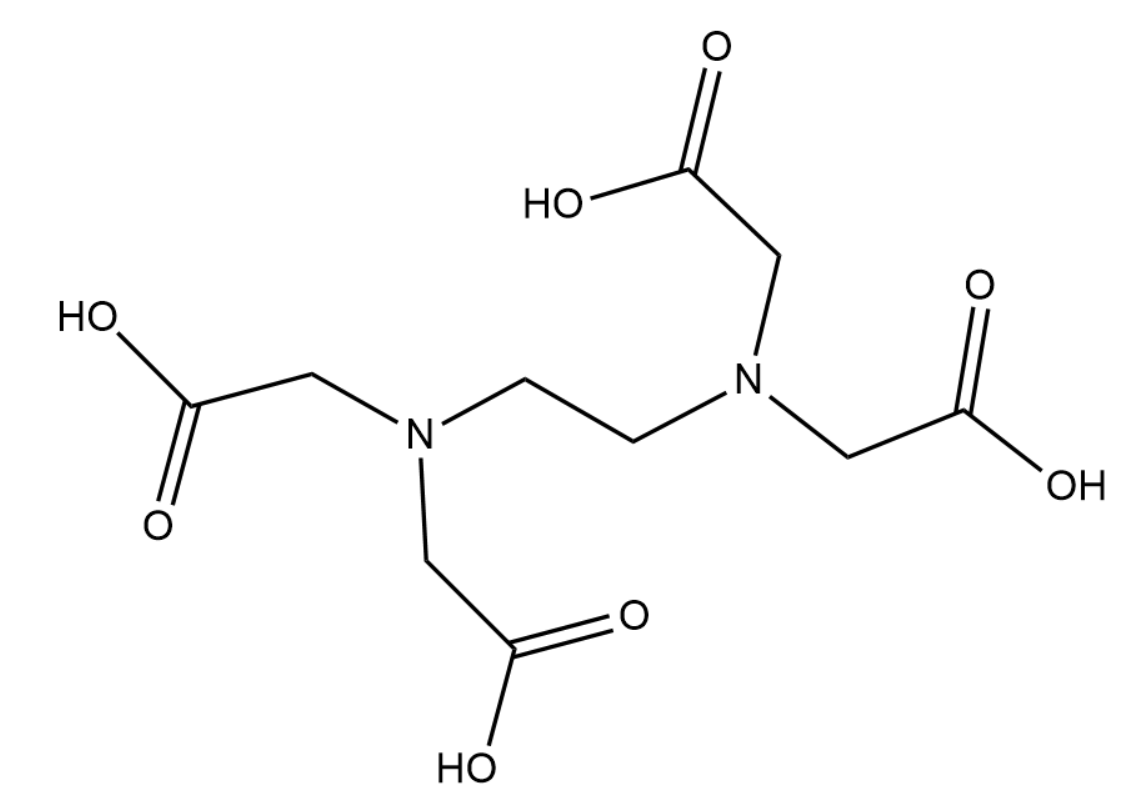
Complete answer:
From the name itself it is clear that EDTA is a weak acid. It behaves as an amino polyprotic acid. Due to the presence of the four carbonyl groups it can lose that hydrogen thus gaining a negatively charged \[CO{O^ - }\] group in each of the four acid groups. Thus EDTA is mildly acidic in nature.
EDTA can lose all 4 of its hydrogen and form\[EDT{A^{4 - }}\]. This along with the lone pairs on each nitrogen can form a total of 6 bonds with the metal ion. This is the reason why EDTA is called a hexadentate ligand. The metal-EDTA complex usually has an octahedral geometry.
Note:
Complexometric titration is a form of volumetric analysis that is used mainly to determine metal ions by use of complex-forming reactions. When EDTA is used alongside Eriochrome Black T (a blue indicator) the colour of the solution changes from wine red to blue indicating the presence of metal ions such as\[C{a^{2 + }},M{g^{2 + }}\]. EDTA has a claw-like structure that we use to stick and grab other molecules and thus forms coordination complexes with them.
The structure of EDTA is given below-

Complete answer:
From the name itself it is clear that EDTA is a weak acid. It behaves as an amino polyprotic acid. Due to the presence of the four carbonyl groups it can lose that hydrogen thus gaining a negatively charged \[CO{O^ - }\] group in each of the four acid groups. Thus EDTA is mildly acidic in nature.
EDTA can lose all 4 of its hydrogen and form\[EDT{A^{4 - }}\]. This along with the lone pairs on each nitrogen can form a total of 6 bonds with the metal ion. This is the reason why EDTA is called a hexadentate ligand. The metal-EDTA complex usually has an octahedral geometry.
Note:
Complexometric titration is a form of volumetric analysis that is used mainly to determine metal ions by use of complex-forming reactions. When EDTA is used alongside Eriochrome Black T (a blue indicator) the colour of the solution changes from wine red to blue indicating the presence of metal ions such as\[C{a^{2 + }},M{g^{2 + }}\]. EDTA has a claw-like structure that we use to stick and grab other molecules and thus forms coordination complexes with them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




