
Is \[C{O_2}\] an ionic compound?
Answer
506.4k+ views
Hint: We have to know that an ionic bond is formed mostly between metals and non-metals. For example: Sodium chloride formed by an ionic bond between sodium and chlorine ions. And the compounds having ionic bonds are known as Ionic compounds.
Complete answer:
As discussed above, an ionic compound is a compound that is mostly formed between a metal atom and a non-metal atom. No, \[C{O_2}\] is not an ionic compound. Meanwhile, \[C{O_2}\] is a compound that is formed between two non-metal atoms (carbon and oxygen) thus giving it a covalent nature. In carbon dioxide one carbon atom will share its four electrons with two electrons from each of the oxygen atoms. With this, all three atoms will have full 8 electrons and there is a formation of two double bonds $\left( {O = C = O} \right)$.
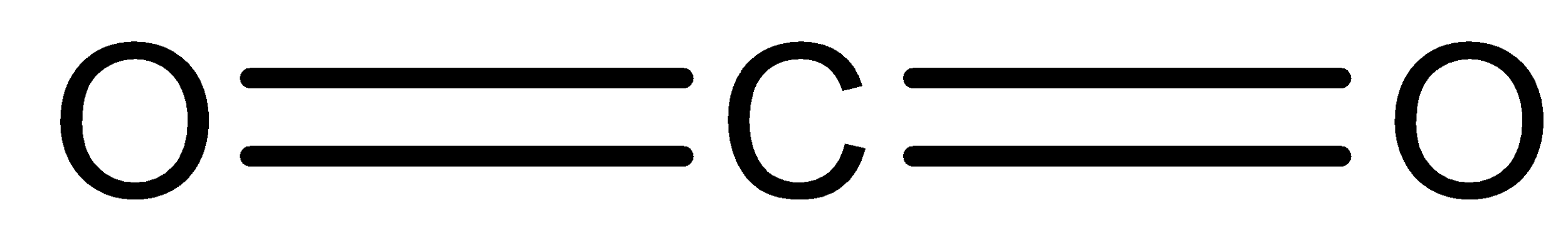
Carbon and oxygen both are non-metals. Carbon has four valence electrons while oxygen has six valence electrons. The bonds holding carbon and oxygen atoms together are double bonds. On the other hand when we look for a covalent bond, it is formed between two non-metallic elements having same or different electronegativity. Since carbon and oxygen both are non-metals thus carbon dioxide is a covalent compound, as it has covalent bond in them.
Note:
As we know that the non-metals have high electron affinity and high electronegative value whereas metals are electropositive in nature. Carbon dioxide is an organic compound since it has a carbon atom. Covalent bonds can be formed by sharing electrons of the same kind of atoms such as Hydrogen molecule also by sharing electrons of different kinds of atoms such as Carbon dioxide molecule.
Complete answer:
As discussed above, an ionic compound is a compound that is mostly formed between a metal atom and a non-metal atom. No, \[C{O_2}\] is not an ionic compound. Meanwhile, \[C{O_2}\] is a compound that is formed between two non-metal atoms (carbon and oxygen) thus giving it a covalent nature. In carbon dioxide one carbon atom will share its four electrons with two electrons from each of the oxygen atoms. With this, all three atoms will have full 8 electrons and there is a formation of two double bonds $\left( {O = C = O} \right)$.
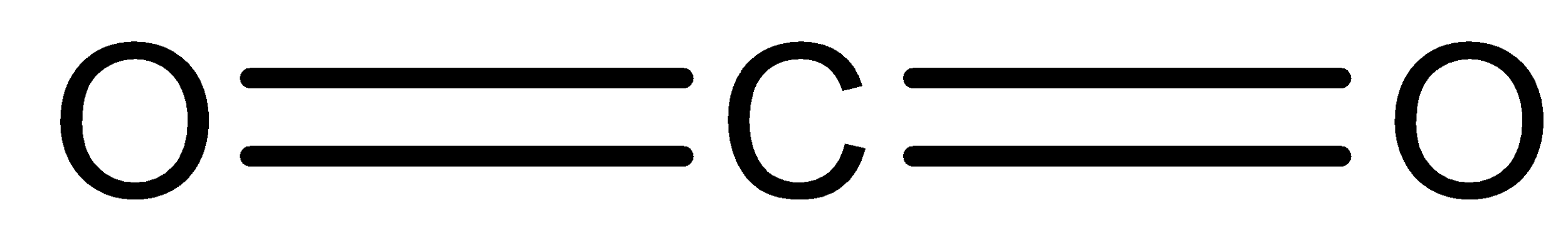
Carbon and oxygen both are non-metals. Carbon has four valence electrons while oxygen has six valence electrons. The bonds holding carbon and oxygen atoms together are double bonds. On the other hand when we look for a covalent bond, it is formed between two non-metallic elements having same or different electronegativity. Since carbon and oxygen both are non-metals thus carbon dioxide is a covalent compound, as it has covalent bond in them.
Note:
As we know that the non-metals have high electron affinity and high electronegative value whereas metals are electropositive in nature. Carbon dioxide is an organic compound since it has a carbon atom. Covalent bonds can be formed by sharing electrons of the same kind of atoms such as Hydrogen molecule also by sharing electrons of different kinds of atoms such as Carbon dioxide molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




