
Is Biphenyl a planar?
Answer
541.8k+ views
Hint:The structure would be influenced by the hybridization and other factors which can affect the effects of hybridisation too.
According to the hybridisation of carbon connecting the phenyl groups i.e. $s{{p}^{2}}$ ; the structure should be planar.
Complete step-by-step answer:Let us study Biphenyl in detail;
Biphenyl is an aromatic hydrocarbon compound with molecular formula as ${{\left( {{C}_{6}}{{H}_{5}} \right)}_{2}}$ . It is an intermediate for the production of many other organic compounds as crop protection products and plastics. Mostly insoluble in water but soluble in many organic solvents.
The structure of the compound is as follows;
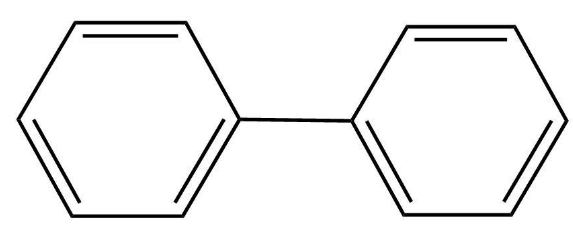
Though the structure represents the planar structure, it is not. This is because the hydrogen present in the compound at the ortho positions (of both the phenyl groups) does not allow the rotation about the central bond joining the two phenyl rings. This is completely due to steric hindrance.
Hence, there can be some deviation from the planar structure.
Therefore, Biphenyl is not planar.
Note:Biphenyl can also be called as diphenyl, phenyl benzene or limonene.
Along with the steric hindrance, the phenyl groups are atropisomers of each other i.e. the conformational isomers which can be isolated at high temperature. This is also a factor for its non-planar structure.
According to the hybridisation of carbon connecting the phenyl groups i.e. $s{{p}^{2}}$ ; the structure should be planar.
Complete step-by-step answer:Let us study Biphenyl in detail;
Biphenyl is an aromatic hydrocarbon compound with molecular formula as ${{\left( {{C}_{6}}{{H}_{5}} \right)}_{2}}$ . It is an intermediate for the production of many other organic compounds as crop protection products and plastics. Mostly insoluble in water but soluble in many organic solvents.
The structure of the compound is as follows;

Though the structure represents the planar structure, it is not. This is because the hydrogen present in the compound at the ortho positions (of both the phenyl groups) does not allow the rotation about the central bond joining the two phenyl rings. This is completely due to steric hindrance.
Hence, there can be some deviation from the planar structure.
Therefore, Biphenyl is not planar.
Note:Biphenyl can also be called as diphenyl, phenyl benzene or limonene.
Along with the steric hindrance, the phenyl groups are atropisomers of each other i.e. the conformational isomers which can be isolated at high temperature. This is also a factor for its non-planar structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




