
Ions, when an ionic compound is dissolved in water, can best be described as:
A. Hydrated molecules only
B. Dehydrated ions and molecules
C. Both hydrated molecules and hydrated ions
D. Neither hydrated ions nor hydrated molecules
E. Hydrated ions only
Answer
588.3k+ views
Hint: We know that the ionic compounds are those compounds that are usually made up of ions and give both the charges that are positive and negative.
Complete step by step answer:
As we know that, the ionic compounds are dissolved in water for the reason that the water molecules hydrate their ions. When the ionic compounds dissolve in water, the water molecules stabilize the ions that are generally caused by breaking the ionic bond. They only do this for hydrating the produced ions.
The water is one of the polar molecules. It usually has the permanent dipole moment. The oxygen atom has a partial negative charge, and the hydrogen atom has a partial positive charge. When the ionic constituent is placed in water, then the water molecules appeal to the positive and negative ions of the crystal. The positive ions are covered with oxygen atoms, and they have several water molecules of a positive charge.
Similarly, the negative ions are covered with hydrogen atoms, and they have several water molecules of the negative charge. The water molecules reduce the attractions between the ionic ions. So, the ions are hydrated. The diagram of hydrate ions is given below:
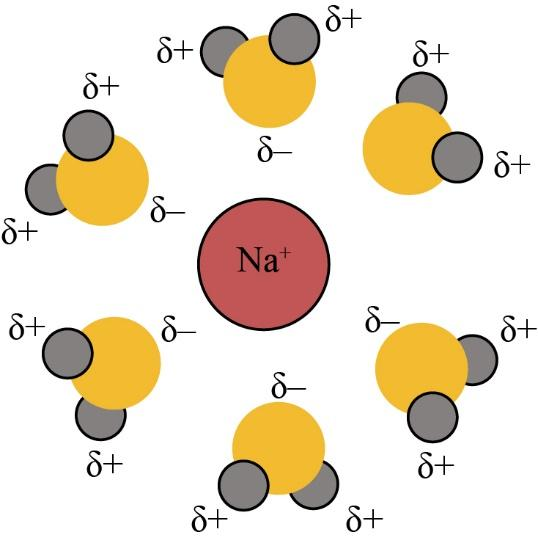
So, the correct answer is “Option E”.
Note:
The ionic compounds are dissolved in water then their ions are hydrated in it. The ionic compounds are water-soluble.
Complete step by step answer:
As we know that, the ionic compounds are dissolved in water for the reason that the water molecules hydrate their ions. When the ionic compounds dissolve in water, the water molecules stabilize the ions that are generally caused by breaking the ionic bond. They only do this for hydrating the produced ions.
The water is one of the polar molecules. It usually has the permanent dipole moment. The oxygen atom has a partial negative charge, and the hydrogen atom has a partial positive charge. When the ionic constituent is placed in water, then the water molecules appeal to the positive and negative ions of the crystal. The positive ions are covered with oxygen atoms, and they have several water molecules of a positive charge.
Similarly, the negative ions are covered with hydrogen atoms, and they have several water molecules of the negative charge. The water molecules reduce the attractions between the ionic ions. So, the ions are hydrated. The diagram of hydrate ions is given below:

So, the correct answer is “Option E”.
Note:
The ionic compounds are dissolved in water then their ions are hydrated in it. The ionic compounds are water-soluble.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




