
Ionic hydrides are formed by:
(A) Transition metals
(B) Elements of very high electropositivity
(C) Elements of very low electropositivity
(D) Metalloids
Answer
601.5k+ views
Hint: Stoichiometric compounds of hydrogen are formed with those elements of the periodic table whose valence shells have one or two electrons and do not form coordination complexes too. (stoichiometric means relative moles of different reactants react with each other in a balanced chemical equation).
Complete step by step answer:
The elements which form ionic hydrides are the elements of the s-block of the periodic table which are highly electropositive in nature.
Some of the properties of the ionic hydrides are – crystalline structure, non-volatile, non-conductor in solid state, white, colourless, high melting and boiling point and decomposed by water, alcohol very easily.
These are formed by the alkali and alkaline earth metals (Gr-I and Gr-II) except \[Be\]and \[Mg\].
\[2Na+\text{ }{{H}_{2}}\text{ }\xrightarrow{573K}\text{ 2}NaH\]
\[2K+\text{ }{{H}_{2}}\text{ }\xrightarrow{673K}\text{ 2}KH\]
\[Ca+\text{ }{{H}_{2}}\text{ }\xrightarrow{1073K}\text{ }Ca{{H}_{2}}\]
\[Sr+\text{ }{{H}_{2}}\text{ }\xrightarrow{1173K}\text{ }Sr{{H}_{2}}\]
So, the correct option is B.
Additional information:
> Transition metals form covalent hydrides which are also known as interstitial hydrides.
> There are three types of hydrides-
1) Ionic hydrides (salt-like hydrides)
2) Covalent hydrides (molecular hydrides)
3) Metallic hydrides (non-stoichiometric hydrides)
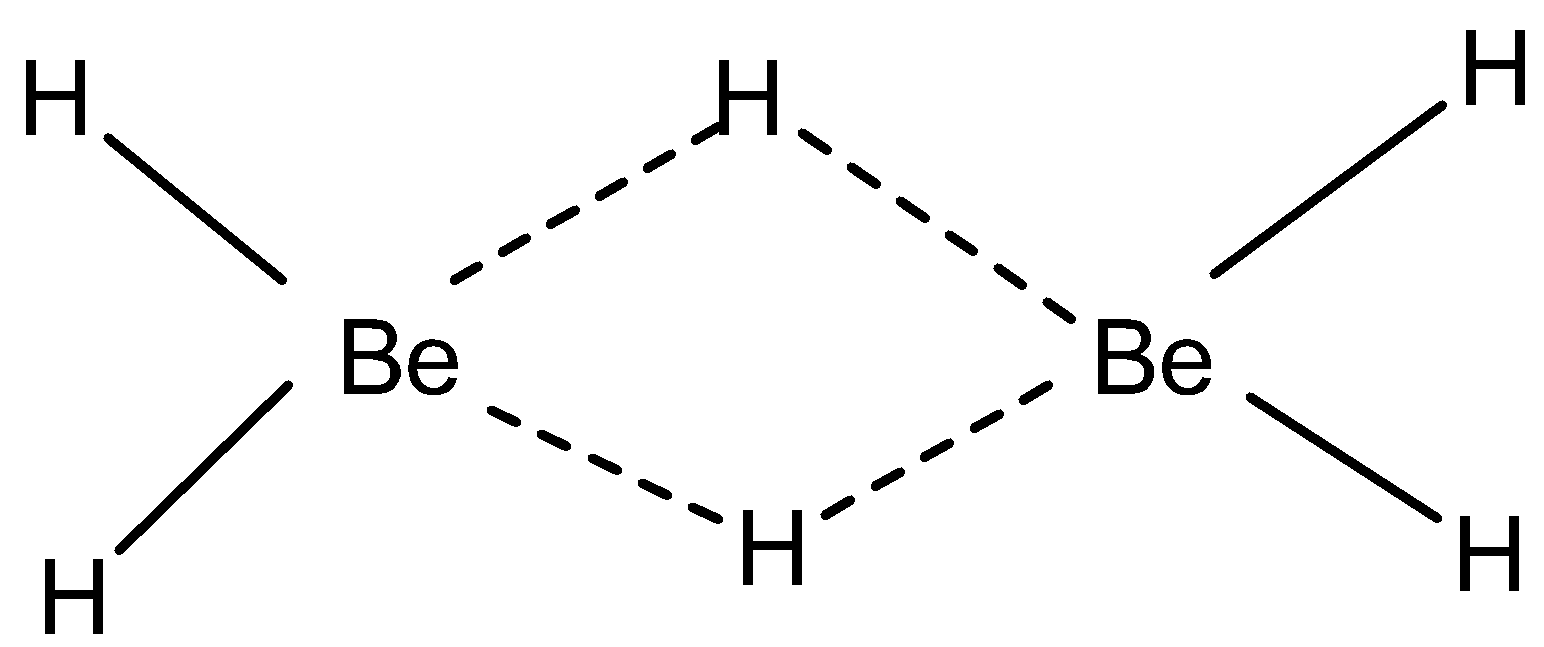
Note: \[LiH\] decomposes at \[400-{{500}^{\circ }}C\] (exception). \[Mg\] and \[Be\] form polymeric hydrides because of the small electronegativity difference. Because of their small size, these metals are covalent in nature and form bridging or polymeric hydrides due to covalent bonding.
\[Be\] occurs as \[{{\text{(}Be{{H}_{2}})}_{n}}\] also known as diborane. \[Mg{{H}_{2}}\] occurs as 3-D structure also known as rutile structure.
Complete step by step answer:
The elements which form ionic hydrides are the elements of the s-block of the periodic table which are highly electropositive in nature.
Some of the properties of the ionic hydrides are – crystalline structure, non-volatile, non-conductor in solid state, white, colourless, high melting and boiling point and decomposed by water, alcohol very easily.
These are formed by the alkali and alkaline earth metals (Gr-I and Gr-II) except \[Be\]and \[Mg\].
\[2Na+\text{ }{{H}_{2}}\text{ }\xrightarrow{573K}\text{ 2}NaH\]
\[2K+\text{ }{{H}_{2}}\text{ }\xrightarrow{673K}\text{ 2}KH\]
\[Ca+\text{ }{{H}_{2}}\text{ }\xrightarrow{1073K}\text{ }Ca{{H}_{2}}\]
\[Sr+\text{ }{{H}_{2}}\text{ }\xrightarrow{1173K}\text{ }Sr{{H}_{2}}\]
So, the correct option is B.
Additional information:
> Transition metals form covalent hydrides which are also known as interstitial hydrides.
> There are three types of hydrides-
1) Ionic hydrides (salt-like hydrides)
2) Covalent hydrides (molecular hydrides)
3) Metallic hydrides (non-stoichiometric hydrides)
Note: \[LiH\] decomposes at \[400-{{500}^{\circ }}C\] (exception). \[Mg\] and \[Be\] form polymeric hydrides because of the small electronegativity difference. Because of their small size, these metals are covalent in nature and form bridging or polymeric hydrides due to covalent bonding.
\[Be\] occurs as \[{{\text{(}Be{{H}_{2}})}_{n}}\] also known as diborane. \[Mg{{H}_{2}}\] occurs as 3-D structure also known as rutile structure.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




