
When iodoform is heated with silver powder, it forms:
A.Methane
B.Ethane
C.Acetylene
D.Ethylene
Answer
564.9k+ views
Hint: We have to know that the iodoform (also known as triiodomethane) molecular formula is $CH{I_3}$ . It is a volatile organic compound with yellow colour and it is a crystalline solid. Iodoform structurally resembles chloroform (molecular formula is $CHC{l_3}$ ) and also resembles chemical properties of chloroform but compared to chloroform, iodoform is less stable.
Complete answer:
We need to remember that one of the methods of preparation of Iodoform is the reaction of ethanol with an iodine (excess) and sodium carbonate is,
${C_2}{H_5}OH + 4{I_2} + 3N{a_2}C{O_3} \to CH{I_3} + HCOONa + 5NaI + 3C{O_2} \uparrow + 2{H_2}O$
We must know that the silver powder is prepared by reducing silver nitrate by glycerol (acts as solvent in this reaction) at below $175^\circ C$ . In this method high purity fine silver powder is obtained.
We can write the chemical reaction of iodoform is heated with silver powder follows as,
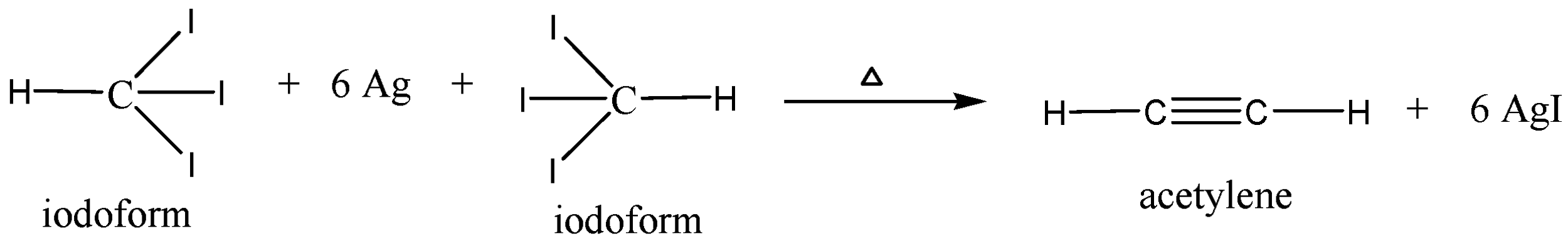
We must know that when two moles iodoform is heated with six moles of silver powder it gives one mole of acetylene (also known as ethyne) and six moles of silver iodide $(AgI)$ . In the above chemical reaction iodoform is reduced and silver was acts as reducing agent in the reaction
So, the correct answer is “Option C”.
Additional information:
We need to remember that the iodoform test is used for the detection of the structural feature of alcohols. Iodoform is used as antiseptic. Iodine is liberated from Iodoform because Iodoform is used as antiseptic in cuts and wounds.
Note:
We must know that in iodoform, the free iodine is liberated by the action of moisture, air and light. Iodoform also decomposes when heating, to give iodine vapours. Iodoform does not oxidize with phosgene light compounds and also does not react with acetone. Silver powder is used to help improve the lack of large and congested pores, also silver powder is used for absorbing excess oil and draws out blackheads.
Complete answer:
We need to remember that one of the methods of preparation of Iodoform is the reaction of ethanol with an iodine (excess) and sodium carbonate is,
${C_2}{H_5}OH + 4{I_2} + 3N{a_2}C{O_3} \to CH{I_3} + HCOONa + 5NaI + 3C{O_2} \uparrow + 2{H_2}O$
We must know that the silver powder is prepared by reducing silver nitrate by glycerol (acts as solvent in this reaction) at below $175^\circ C$ . In this method high purity fine silver powder is obtained.
We can write the chemical reaction of iodoform is heated with silver powder follows as,

We must know that when two moles iodoform is heated with six moles of silver powder it gives one mole of acetylene (also known as ethyne) and six moles of silver iodide $(AgI)$ . In the above chemical reaction iodoform is reduced and silver was acts as reducing agent in the reaction
So, the correct answer is “Option C”.
Additional information:
We need to remember that the iodoform test is used for the detection of the structural feature of alcohols. Iodoform is used as antiseptic. Iodine is liberated from Iodoform because Iodoform is used as antiseptic in cuts and wounds.
Note:
We must know that in iodoform, the free iodine is liberated by the action of moisture, air and light. Iodoform also decomposes when heating, to give iodine vapours. Iodoform does not oxidize with phosgene light compounds and also does not react with acetone. Silver powder is used to help improve the lack of large and congested pores, also silver powder is used for absorbing excess oil and draws out blackheads.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




