
What is an interhalogen compound? Describe the geometry of $A{B_3}{E_2}$ type chlorine-fluorine interhalogen compounds.
Answer
542.4k+ views
Hint: An interhalogen compound is a molecule which incorporates or more exclusive halogen atoms (fluorine, chlorine, bromine, iodine, or astatine) and no atoms of factors from another group.
Most interhalogen compounds acknowledged are binary (composed of only wonderful elements). Their formulae are typically$X{Y_n}$, in which $n = 1,3,5,7andX$is the less electronegative of the two halogens. The price of n in interhalogens is always peculiar, because of the atypical valence of halogens. They are all liable to hydrolysis, and ionize to offer rise to polyhalogen ions. Those formed with astatine have a totally quick half of-lifestyles because of astatine being intensely radioactive.
Complete answer:
Interhalogen compound-
Interhalogen compounds are compounds formed whilst halogen organization elements react with each other. In different phrases, it is a molecule which consists of two or more distinct elements of the group $17$. There are four varieties of interhalogen compounds:
1. Diatomic interhalogens ($AX$)
2. Tetratomic interhalogens ($A{X_3}$)
3. Hexatomic interhalogens ($A{X_5}$)
4. Octatomic interhalogens ($A{X_7}$)
A halogen with huge size and excessive electropositivity reacts with a detail of group 17 with small length and lower electro positivity. As the ratio of the radius of larger and smaller halogen will increase, the variety of atoms in a molecule additionally increases.
Geometry of $A{B_3}{E_2}$ type chlorine-fluorine interhalogen compounds-
$Cl{F_3}$ Chlorine Trifluoride
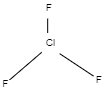
Chlorine trifluoride has five areas of electron density around the relevant chlorine atom (3 bonds and a couple of lone pairs). These are organized in a trigonal bipyramidal form with a one hundred seventy five° F(axial)-Cl-F(axial) bond angle.
The lone pairs take equatorial positions because they call for extra space than the bonds. The end result is a T-shape molecule.
Note:
T-shaped molecular geometry describes the structures of some molecules wherein a central atom has three ligands. Ordinarily, three-coordinated compounds adopt trigonal planar or pyramidal geometries. Examples of T-fashioned molecules are the halogen trifluorides, which includes $Cl{F_3}$.
Most interhalogen compounds acknowledged are binary (composed of only wonderful elements). Their formulae are typically$X{Y_n}$, in which $n = 1,3,5,7andX$is the less electronegative of the two halogens. The price of n in interhalogens is always peculiar, because of the atypical valence of halogens. They are all liable to hydrolysis, and ionize to offer rise to polyhalogen ions. Those formed with astatine have a totally quick half of-lifestyles because of astatine being intensely radioactive.
Complete answer:
Interhalogen compound-
Interhalogen compounds are compounds formed whilst halogen organization elements react with each other. In different phrases, it is a molecule which consists of two or more distinct elements of the group $17$. There are four varieties of interhalogen compounds:
1. Diatomic interhalogens ($AX$)
2. Tetratomic interhalogens ($A{X_3}$)
3. Hexatomic interhalogens ($A{X_5}$)
4. Octatomic interhalogens ($A{X_7}$)
A halogen with huge size and excessive electropositivity reacts with a detail of group 17 with small length and lower electro positivity. As the ratio of the radius of larger and smaller halogen will increase, the variety of atoms in a molecule additionally increases.
Geometry of $A{B_3}{E_2}$ type chlorine-fluorine interhalogen compounds-
$Cl{F_3}$ Chlorine Trifluoride

Chlorine trifluoride has five areas of electron density around the relevant chlorine atom (3 bonds and a couple of lone pairs). These are organized in a trigonal bipyramidal form with a one hundred seventy five° F(axial)-Cl-F(axial) bond angle.
The lone pairs take equatorial positions because they call for extra space than the bonds. The end result is a T-shape molecule.
Note:
T-shaped molecular geometry describes the structures of some molecules wherein a central atom has three ligands. Ordinarily, three-coordinated compounds adopt trigonal planar or pyramidal geometries. Examples of T-fashioned molecules are the halogen trifluorides, which includes $Cl{F_3}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




