
Indicate the alkyl halides one of the following which can undergo \[S{{N}^{1}}\] reaction.
a.
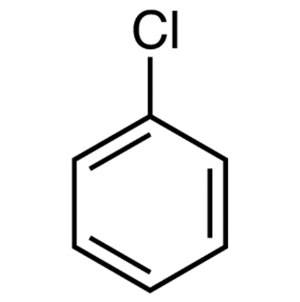
b.
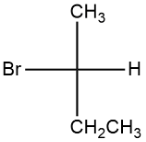
c. \[C{{H}_{2}}=CHCl\]
d. \[C{{H}_{2}}=CHC{{H}_{2}}Cl\]


Answer
592.2k+ views
Hint: \[S{{N}^{1}}\] is a nucleophilic substitution reaction where the rate determining step is unimolecular. The reaction involves the formation of an intermediate carbocation. It is generally seen in tertiary or secondary alkyl halides with secondary or tertiary alcohols.
Complete answer:
\[S{{N}^{1}}\] reaction depends on the concentration of only one reactant. It occurs in two steps:
In step one the bond undergoes slow cleavage to form carbocation and in step two it completes the substitution reaction.
The order for \[S{{N}^{1}}\] reactions tertiary halide > secondary halide > primary halide.
Now in the given question the first compound which is chlorobenzene. Chlorine being an electron withdrawing group is still too weak to activate the ring towards substitution, and thus no reaction will occur. nucleophilic aromatic reactions generally require a very strong electron withdrawing group.
The second compound, 2-bromobutane, the halogen group here is an effective leaving group. \[S{{N}^{2}}\] is generally favorable for the nucleophilic substitution reaction of
2-bromobutane as it will demand more external energy for its complete reaction. Hence, it shows \[S{{N}^{2}}\] reaction.
The third compound, Vinyl chloride is generally unreactive towards nucleophilic substitution reaction due to resonance. The lone pair of electrons on chlorine is in resonance with the C-C double bond, due to which there will be a partial double bond character in C-Cl bond.
The fourth compound, allyl chloride, mostly shows \[S{{N}^{1}}\] reaction as it involves the formation of intermediate carbocation. carbocation formed from allyl chloride achieves stability by resonance.
Therefore, from the above statements we can conclude allyl chloride is more reactive towards \[S{{N}^{1}}\] reaction,So, the correct answer is “Option D”.
Note: \[S{{N}^{2}}\] is a nucleophilic substitution reaction that depends on two components. They are bimolecular with simultaneous bond-making and bond-breaking steps.
The reactivity of \[S{{N}^{2}}\] halides: primary> secondary> tertiary
Complete answer:
\[S{{N}^{1}}\] reaction depends on the concentration of only one reactant. It occurs in two steps:
In step one the bond undergoes slow cleavage to form carbocation and in step two it completes the substitution reaction.
The order for \[S{{N}^{1}}\] reactions tertiary halide > secondary halide > primary halide.
Now in the given question the first compound which is chlorobenzene. Chlorine being an electron withdrawing group is still too weak to activate the ring towards substitution, and thus no reaction will occur. nucleophilic aromatic reactions generally require a very strong electron withdrawing group.
The second compound, 2-bromobutane, the halogen group here is an effective leaving group. \[S{{N}^{2}}\] is generally favorable for the nucleophilic substitution reaction of
2-bromobutane as it will demand more external energy for its complete reaction. Hence, it shows \[S{{N}^{2}}\] reaction.
The third compound, Vinyl chloride is generally unreactive towards nucleophilic substitution reaction due to resonance. The lone pair of electrons on chlorine is in resonance with the C-C double bond, due to which there will be a partial double bond character in C-Cl bond.
The fourth compound, allyl chloride, mostly shows \[S{{N}^{1}}\] reaction as it involves the formation of intermediate carbocation. carbocation formed from allyl chloride achieves stability by resonance.
Therefore, from the above statements we can conclude allyl chloride is more reactive towards \[S{{N}^{1}}\] reaction,So, the correct answer is “Option D”.
Note: \[S{{N}^{2}}\] is a nucleophilic substitution reaction that depends on two components. They are bimolecular with simultaneous bond-making and bond-breaking steps.
The reactivity of \[S{{N}^{2}}\] halides: primary> secondary> tertiary
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




