
What is the index of hydrogen deficiency in the molecule \[{{{C}}_{{{12}}}}{{{H}}_{{{17}}}}{{NO}}\]?
1) \[{{4}}\]
2) \[{{5}}\]
3) \[{{6}}\]
4) \[{{7}}\]
Answer
558.3k+ views
Hint:Index of hydrogen deficiency provides the number of multiple bonds and rings are present in the structure of the respective compound. In this formula we substituted a number of carbons, hydrogen, nitrogen and oxygen atoms present in the given compound.
Complete step by step answer:
Index of hydrogen deficiency can easily be calculated by drawing the structure of the compound. When we have the structure, we can count the number of multiple bonds and rings and that gives us the IHD count. But in some cases, we may not be aware of the structure of the compound. In these situations, we can use a simple formula for finding the hydrogen deficiency index.
If you are having a molecular formula of a compound like this \[{{{C}}_{{c}}}{{{H}}_{{h}}}{{{N}}_{{n}}}{{{O}}_{{o}}}{{{X}}_{{x}}}\], then we can write the formula as follows;
\[{{IHD = 0}}{{.5 \times [2c + 2 - h - x + n]}}\] ………………. (1)
Here we have the compound as \[{{{C}}_{{{12}}}}{{{H}}_{{{17}}}}{{NO}}\]which is considered to be an aromatic amine.
From the above equation we can have the values of c=12, h=17, n=1, o=1 and x=0
Substituting these values in the equation (1), we have
$ {{IHD = 0}}{{.5 \times [2 \times 12 + 2 - 17 - 0 + 1]}} \\
{{ = 0}}{{.5 \times [24 - 14]}} \\
{{ = 0}}{{.5 \times [10]}} \\
{{ = 5}} $
This is the simplest way of finding out the index of hydrogen deficiency of a compound.
We can also find out it from the structure of the compound
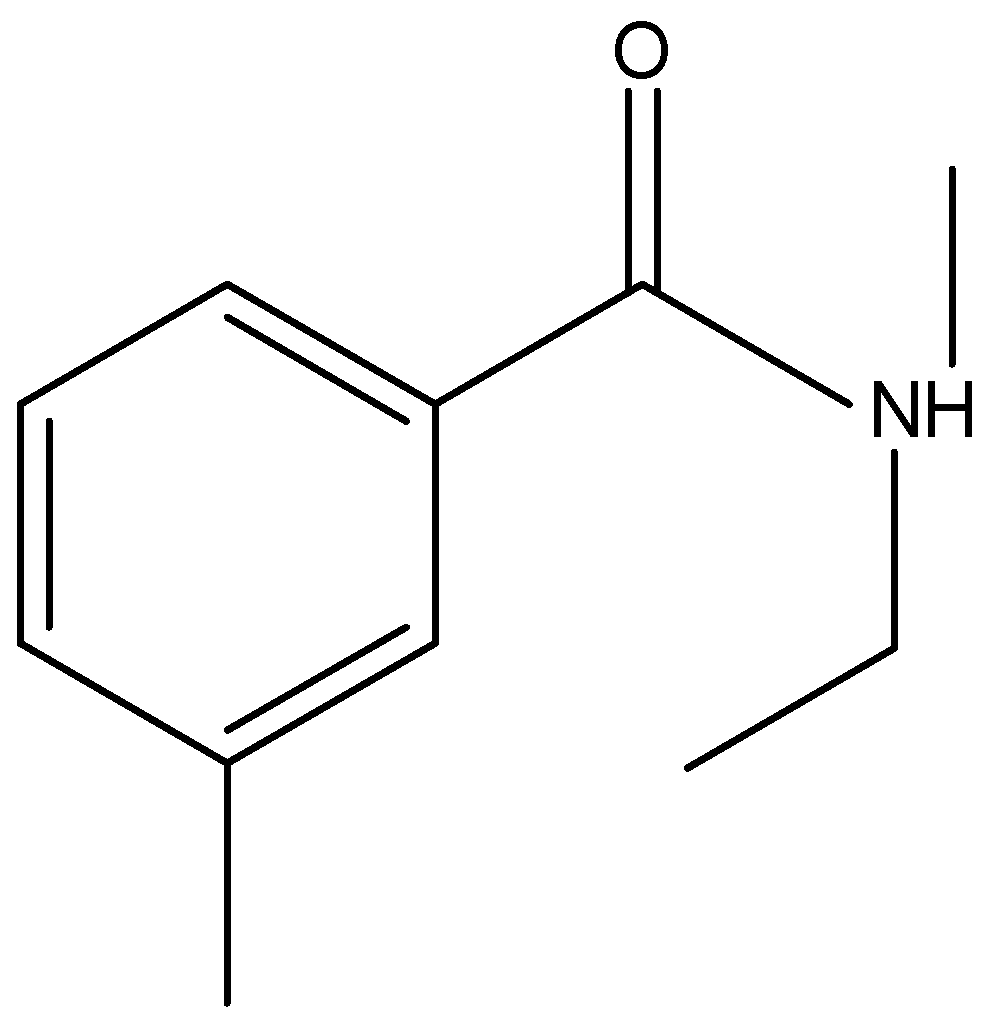
\[{{{C}}_{{{12}}}}{{{H}}_{{{17}}}}{{NO}}\]
Here we can see four multiple bonds and a ring and the total value is five.
Therefore, the index of hydrogen deficiency or IHD of \[{{{C}}_{{{12}}}}{{{H}}_{{{17}}}}{{NO}}\] is five
So, the correct answer is Option 2.
Note: The compound \[{{{C}}_{{{12}}}}{{{H}}_{{{17}}}}{{NO}}\] is iso pentedrone. It is having great uses because of its medicinal value.The IHD value indicates the number of multiple bonds and rings in a compound. It can be easily calculated by using the IHD formula.
Complete step by step answer:
Index of hydrogen deficiency can easily be calculated by drawing the structure of the compound. When we have the structure, we can count the number of multiple bonds and rings and that gives us the IHD count. But in some cases, we may not be aware of the structure of the compound. In these situations, we can use a simple formula for finding the hydrogen deficiency index.
If you are having a molecular formula of a compound like this \[{{{C}}_{{c}}}{{{H}}_{{h}}}{{{N}}_{{n}}}{{{O}}_{{o}}}{{{X}}_{{x}}}\], then we can write the formula as follows;
\[{{IHD = 0}}{{.5 \times [2c + 2 - h - x + n]}}\] ………………. (1)
Here we have the compound as \[{{{C}}_{{{12}}}}{{{H}}_{{{17}}}}{{NO}}\]which is considered to be an aromatic amine.
From the above equation we can have the values of c=12, h=17, n=1, o=1 and x=0
Substituting these values in the equation (1), we have
$ {{IHD = 0}}{{.5 \times [2 \times 12 + 2 - 17 - 0 + 1]}} \\
{{ = 0}}{{.5 \times [24 - 14]}} \\
{{ = 0}}{{.5 \times [10]}} \\
{{ = 5}} $
This is the simplest way of finding out the index of hydrogen deficiency of a compound.
We can also find out it from the structure of the compound

\[{{{C}}_{{{12}}}}{{{H}}_{{{17}}}}{{NO}}\]
Here we can see four multiple bonds and a ring and the total value is five.
Therefore, the index of hydrogen deficiency or IHD of \[{{{C}}_{{{12}}}}{{{H}}_{{{17}}}}{{NO}}\] is five
So, the correct answer is Option 2.
Note: The compound \[{{{C}}_{{{12}}}}{{{H}}_{{{17}}}}{{NO}}\] is iso pentedrone. It is having great uses because of its medicinal value.The IHD value indicates the number of multiple bonds and rings in a compound. It can be easily calculated by using the IHD formula.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




