
Incorrect statement about DMG.
A. It is tetradentate ligand.
B. Chelating agent
C. Dioxime of diacetyl
D. In gravimetric determination of Ni is used
Answer
566.7k+ views
Hint: DMG means Dimethylglyoxime. Dimethylglyoxime is a ligand having a capability to bind with metals. Ligands have a capability to form complexes with metals. DMG is used in the precious metal refining process.
Complete step by step solution:
- In the question it is given that to find the incorrect statement regarding DMG among the given options.
- Coming to given options, option A, It is tetradentate ligand. It is incorrect because DMG is bidentate ligand means it can form two bonds with metal. Means DMG has two donor atoms in it.
- Coming to option B, Chelating agent. It is true because DMG forms chelate or complex with metals when it reacts with them.
- Coming to option C, Dioxime of diacetyl. It is also correct because it contains diacetyl in its structure.
- The structure of DMG is as follows.
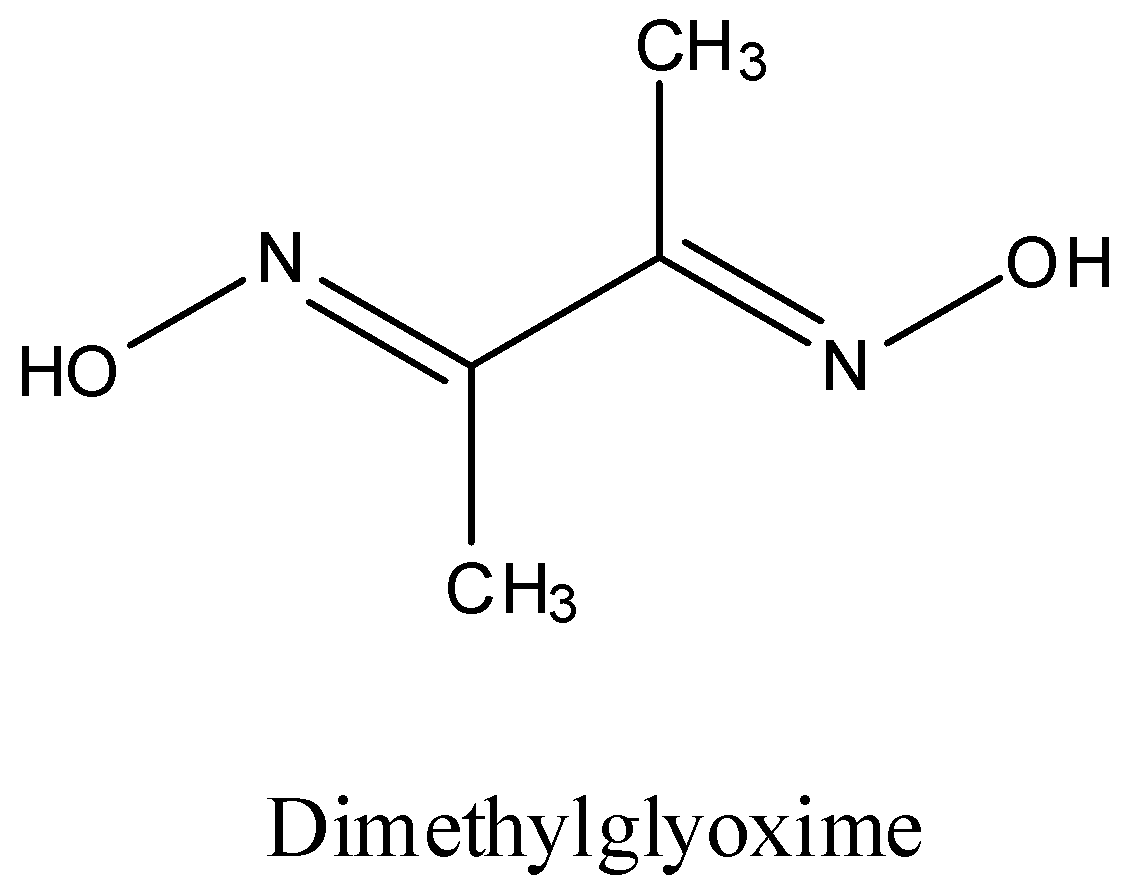
- Coming to option D, In gravimetric determination of Ni is used. It is also correct because in gravimetric analysis to determine the amount of nickel, DMG is going to be used because it forms a complex with nickel.
- Therefore DMG is not a tetradentate ligand. So, the correct answer is “Option A”.
Note: DMG forms complex or chelates with Nickel, Palladium, and cobalt metals. The molecular formula of dimethylglyoxime is ${{C}_{4}}{{H}_{8}}{{N}_{2}}{{O}_{2}}$ . DMG is less soluble in water but highly soluble in a solution of sodium hydroxide and methanol.
Complete step by step solution:
- In the question it is given that to find the incorrect statement regarding DMG among the given options.
- Coming to given options, option A, It is tetradentate ligand. It is incorrect because DMG is bidentate ligand means it can form two bonds with metal. Means DMG has two donor atoms in it.
- Coming to option B, Chelating agent. It is true because DMG forms chelate or complex with metals when it reacts with them.
- Coming to option C, Dioxime of diacetyl. It is also correct because it contains diacetyl in its structure.
- The structure of DMG is as follows.

- Coming to option D, In gravimetric determination of Ni is used. It is also correct because in gravimetric analysis to determine the amount of nickel, DMG is going to be used because it forms a complex with nickel.
- Therefore DMG is not a tetradentate ligand. So, the correct answer is “Option A”.
Note: DMG forms complex or chelates with Nickel, Palladium, and cobalt metals. The molecular formula of dimethylglyoxime is ${{C}_{4}}{{H}_{8}}{{N}_{2}}{{O}_{2}}$ . DMG is less soluble in water but highly soluble in a solution of sodium hydroxide and methanol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




