
Incineration of Municipal waste involves
(a) Oxidation
(b) Deduction
(c) Redoxation
(d) Disintegration
Answer
510.3k+ views
Hint: Change in color of apple when left sliced for a long time is also an example of the required reaction. This is a chemical reaction in which one substance gains oxygen or loses electrons and hydrogen.
Complete answer:
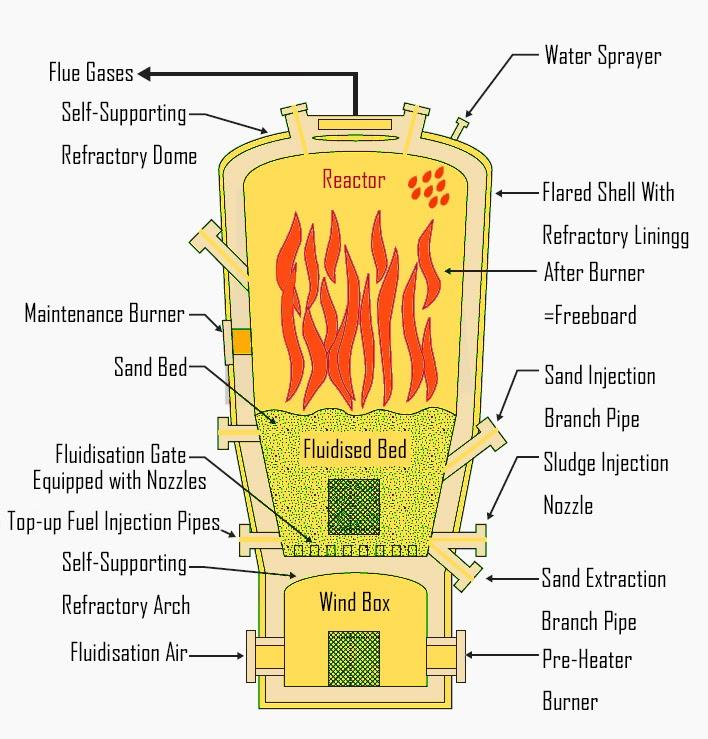
The incineration of municipal waste involves oxidation. The solid waste collected by the municipalities is referred to as municipal waste. This collected waste is later treated.
Non-hazardous disposable materials are also called garbage (municipal waste) or trash, they come from households, institutions, industries, agriculture, etc. This inorganic municipal solid waste is sent for processing or landfill.
Additional Information:
The organic municipal waste is sent for treatment followed by incineration.
-Incineration is the combustion of organic materials contained in solid waste and it involves oxidation.
-It is a waste treatment process that converts the waste into ash, flue gas, and heat.
-Combustion is another name for burning something. It involves a reaction between a fuel and oxygen present in the atmosphere.
-The process that involves reducing something is called deduction.
-The process in which the reducing agent loses electrons and gets oxidized and the oxidizing agent gains electrons and gets reduced is called redoxation.
-The process of disintegration involves breaking down into pieces.
So, the correct answer is,’ Oxidation’.
Note:
-Combustion involves oxidation, which is the loss of electrons or ions by a molecule.
-All the high-temperature waste treatment systems including incineration are called thermal treatment.
-Municipal waste does not contain waste from municipal sewage networks and treatment, and construction and demolition activities.
Complete answer:
The incineration of municipal waste involves oxidation. The solid waste collected by the municipalities is referred to as municipal waste. This collected waste is later treated.
Non-hazardous disposable materials are also called garbage (municipal waste) or trash, they come from households, institutions, industries, agriculture, etc. This inorganic municipal solid waste is sent for processing or landfill.
Additional Information:
The organic municipal waste is sent for treatment followed by incineration.
-Incineration is the combustion of organic materials contained in solid waste and it involves oxidation.
-It is a waste treatment process that converts the waste into ash, flue gas, and heat.
-Combustion is another name for burning something. It involves a reaction between a fuel and oxygen present in the atmosphere.
-The process that involves reducing something is called deduction.
-The process in which the reducing agent loses electrons and gets oxidized and the oxidizing agent gains electrons and gets reduced is called redoxation.
-The process of disintegration involves breaking down into pieces.

So, the correct answer is,’ Oxidation’.
Note:
-Combustion involves oxidation, which is the loss of electrons or ions by a molecule.
-All the high-temperature waste treatment systems including incineration are called thermal treatment.
-Municipal waste does not contain waste from municipal sewage networks and treatment, and construction and demolition activities.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




