
In\[CC{l_4}\], the four valencies of carbon are directed the corners of a:
A.Cube
B.Hexagon
C.Prism
D.Tetrahedron
Answer
578.4k+ views
Hint: We all know that,
Hybridization:
Hybridization is the concept during which atomic orbitals combine to form a new hybridized orbital which successively, influences molecular geometry and bonding properties.
We know that the electrons which are present at the outermost shell of an atom are called valence electrons and the valency of an electron is that the amount of electrons during which atom accepts or donate to form a bond.
Complete step by step answer:
The structure of \[CC{l_4}\] has \[4{\text{ }} + {\text{ }}4\left( 7 \right){\text{ }} = {\text{ }}32\] valence electrons. In this structure, the Carbon atom is at the central position and the remaining all Chlorine atoms are placed around it. Since the central atom carbon has four bonded pairs and \[s{p^3}\] hybridization and the shape of the molecule is tetrahedral. Remaining all the non-bonding electrons are spread out in the structure.
We can draw the structure of carbon tetrachloride as,
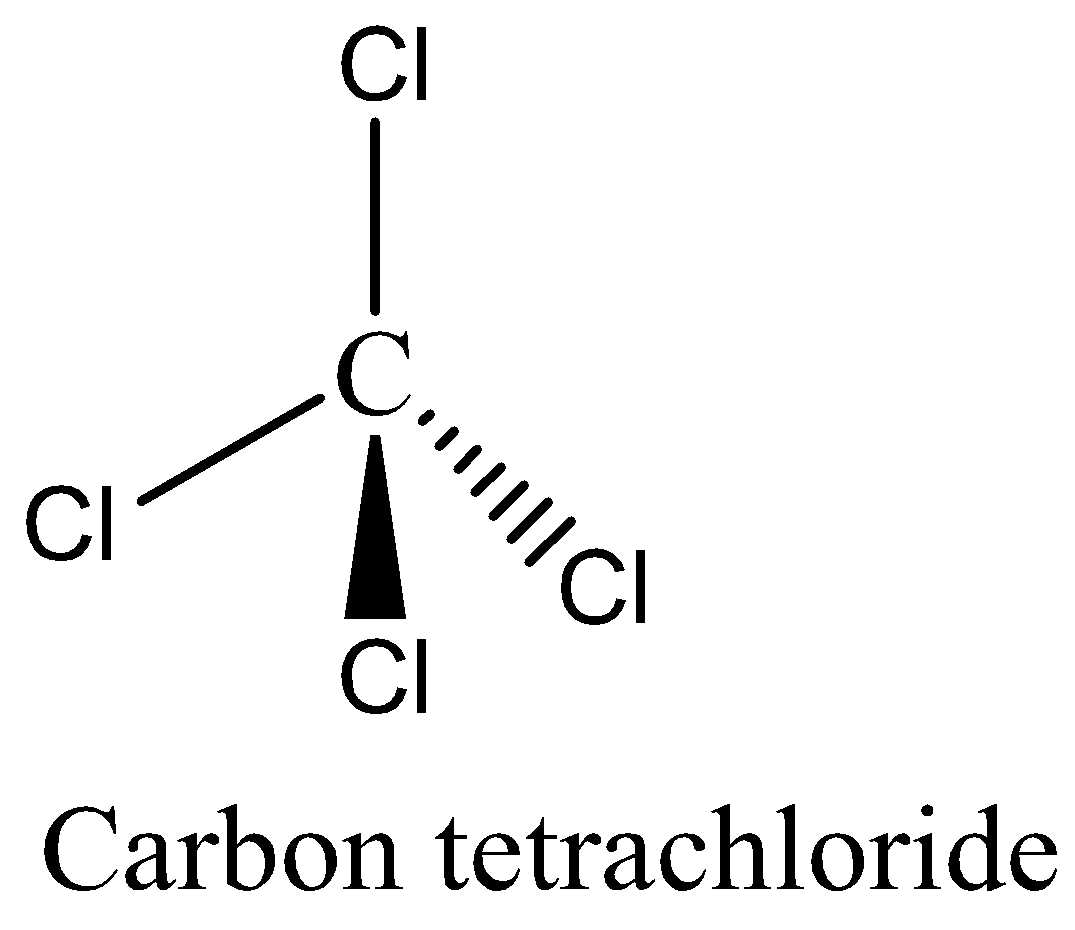
So, the correct answer is Option D.
Note:
Now we discuss about the types of cubic lattice as,
Cubic lattice are classified into three types:
The atom in the simple cubic is shared equally between eight neighboring cubes, and the unit cell therefore contains in total one atom.
Body-centered cubic (bcc) has one lattice point in the center of the unit cell in addition to the eight corner points and the unit cell contains in total two atoms.
Face-centered cubic (FCC) are eight atoms at corners of the unit cell and one atom centered in each of the faces. The atom in the face centered cubic lattice is shared with the adjacent cell.
Hybridization:
Hybridization is the concept during which atomic orbitals combine to form a new hybridized orbital which successively, influences molecular geometry and bonding properties.
We know that the electrons which are present at the outermost shell of an atom are called valence electrons and the valency of an electron is that the amount of electrons during which atom accepts or donate to form a bond.
Complete step by step answer:
The structure of \[CC{l_4}\] has \[4{\text{ }} + {\text{ }}4\left( 7 \right){\text{ }} = {\text{ }}32\] valence electrons. In this structure, the Carbon atom is at the central position and the remaining all Chlorine atoms are placed around it. Since the central atom carbon has four bonded pairs and \[s{p^3}\] hybridization and the shape of the molecule is tetrahedral. Remaining all the non-bonding electrons are spread out in the structure.
We can draw the structure of carbon tetrachloride as,

So, the correct answer is Option D.
Note:
Now we discuss about the types of cubic lattice as,
Cubic lattice are classified into three types:
The atom in the simple cubic is shared equally between eight neighboring cubes, and the unit cell therefore contains in total one atom.
Body-centered cubic (bcc) has one lattice point in the center of the unit cell in addition to the eight corner points and the unit cell contains in total two atoms.
Face-centered cubic (FCC) are eight atoms at corners of the unit cell and one atom centered in each of the faces. The atom in the face centered cubic lattice is shared with the adjacent cell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




