
In which the following inversion is not possible?
A.\[C{H_3}C{H_2} - N{H_2}\]
B.Propan-2-amine
C.
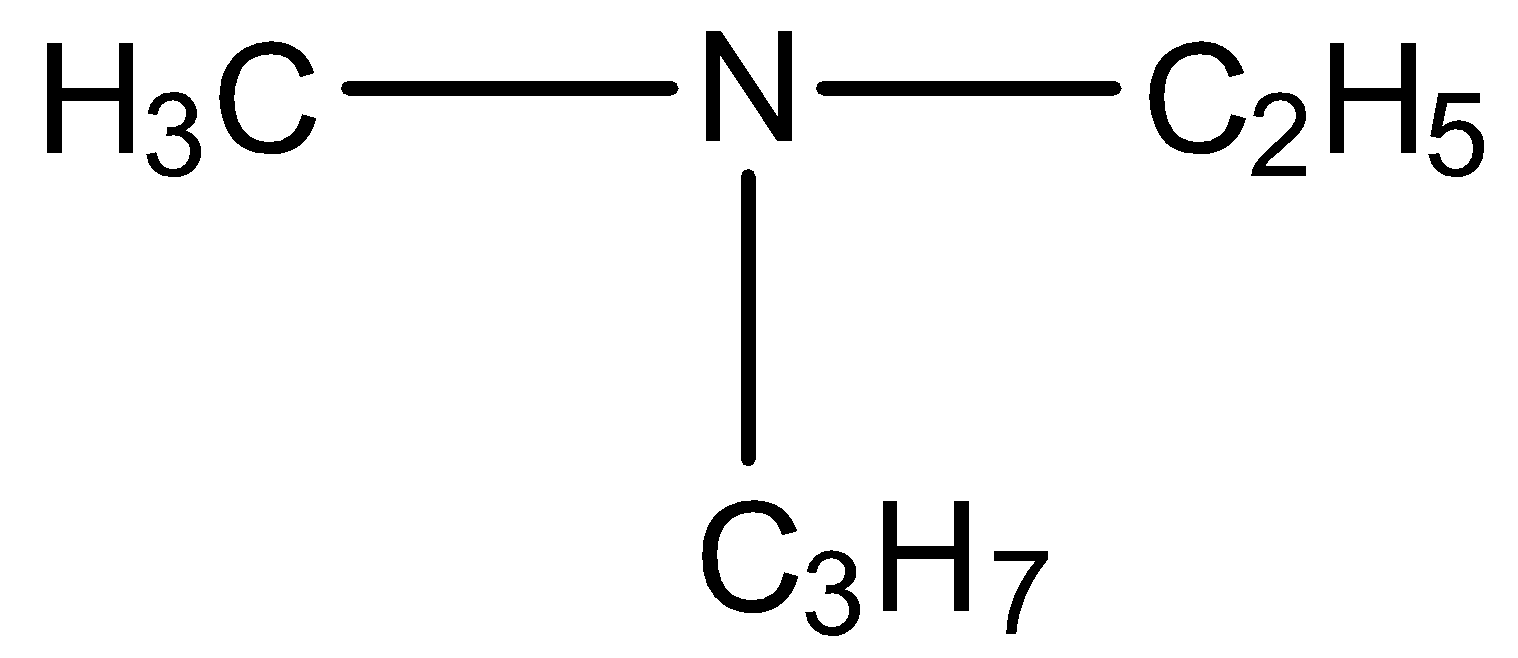
D.
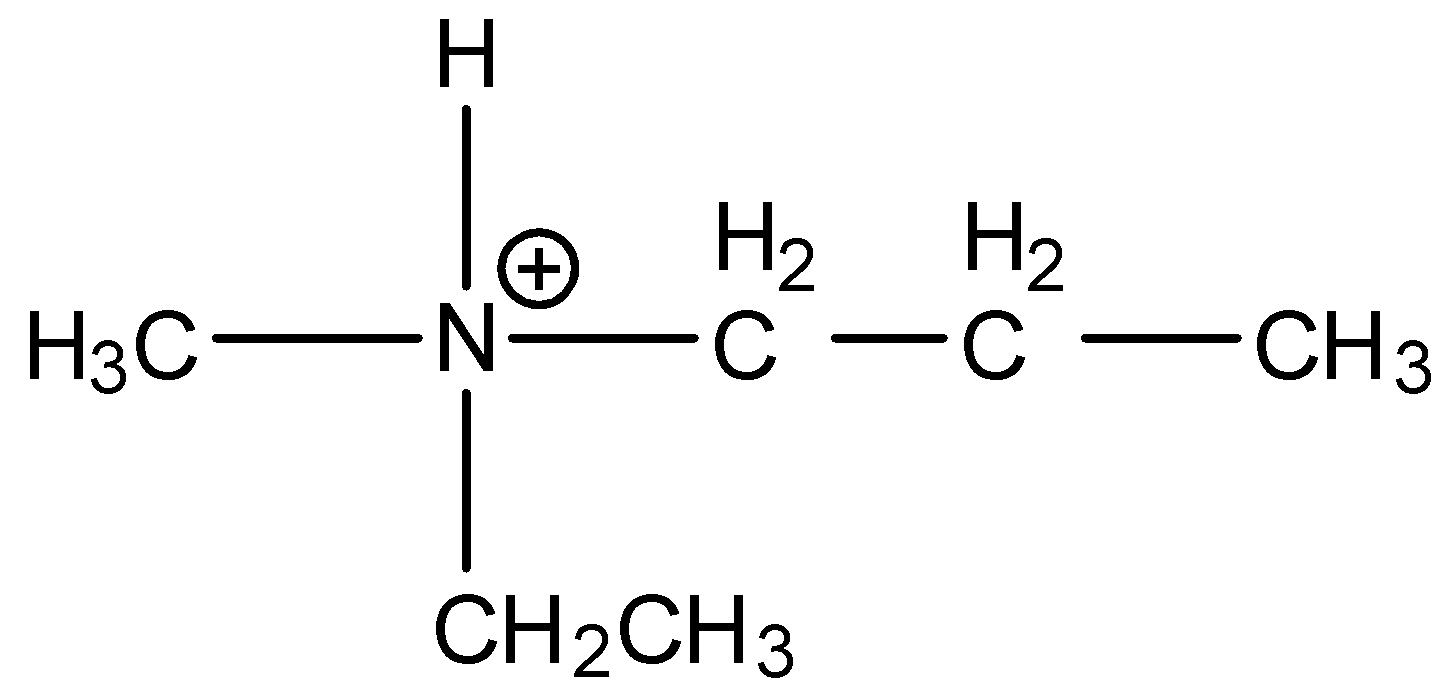


Answer
511.5k+ views
Hint: There is a formation of product with respect to the molecular configuration by the spatial rearrangement of a group of atoms or atoms which is present in a dissymmetric molecule. And the product will be a mirror image of that original compound. During inversion of an atom, its relative configuration should be changed. And here the configuration may change cis to Trans and vice versa.
Complete answer:
In this case, the amine group present in the ethyl amine will show the inversion and there is a formation of methyl amine and ethyl amine. Hence, option (A) is incorrect.
The propan-2-amine is also known as isopropyl amine. And the nitrogen inversion does not take place in isopropyl alcohol. Because, the nitrogen inversion happens only when the R group is linked with nitrogen should be unique. Then the compound should be a chiral compound.
But in the case of propan-2-amine, all the atoms attached with nitrogen are the same. Therefore, inversion is not possible in the case of propan-2-amine. Hence, option (B) is correct.
In this compound all the groups are unique and the inversion occurs. Hence, option (C) is incorrect.
Here, the nitrogen is attached with four different groups and the inversion is possible in this compound. Hence, the option (D) is incorrect.
Note:
Among these given compounds, the inversion is not possible in the case of propan-2-amine. Because, the inversion of nitrogen depends on the groups attached to the nitrogen and all the groups should be unique for the inversion of this compound. But in the case of propan-2-amine, all the groups attached with nitrogen are methylene groups which are the same. Hence, it will not undergo the inversion.
Complete answer:
In this case, the amine group present in the ethyl amine will show the inversion and there is a formation of methyl amine and ethyl amine. Hence, option (A) is incorrect.
The propan-2-amine is also known as isopropyl amine. And the nitrogen inversion does not take place in isopropyl alcohol. Because, the nitrogen inversion happens only when the R group is linked with nitrogen should be unique. Then the compound should be a chiral compound.
But in the case of propan-2-amine, all the atoms attached with nitrogen are the same. Therefore, inversion is not possible in the case of propan-2-amine. Hence, option (B) is correct.
In this compound all the groups are unique and the inversion occurs. Hence, option (C) is incorrect.
Here, the nitrogen is attached with four different groups and the inversion is possible in this compound. Hence, the option (D) is incorrect.
Note:
Among these given compounds, the inversion is not possible in the case of propan-2-amine. Because, the inversion of nitrogen depends on the groups attached to the nitrogen and all the groups should be unique for the inversion of this compound. But in the case of propan-2-amine, all the groups attached with nitrogen are methylene groups which are the same. Hence, it will not undergo the inversion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




