
In which pair of species, the octet rule is not obeyed?
A. $P{F_3}$ and $POC{l_3}$
B. $BC{l_3}$ and $PC{l_5}$
C. $C{F_4}$ and $N{F_3}$
D. $N{H_3}$ and $NC{l_3}$
Answer
576.9k+ views
Hint: We can find the species that does not follow the octet rule by calculating the total number of electrons in the species. The total number of electrons in species is calculated by adding the number of bonded electrons and the number of electrons in the lone pairs.
Complete step by step answer:
We can say a stable arrangement is attained when the atom is surrounded by eight electrons. This octet could be made up of its own electrons and some electrons that are shared. Therefore, an atom continues to form bonds until an octet of electrons is made
Let us now calculate the total number of electrons present in all compounds by adding the electrons in bond pairs and lone pairs.
In the pair (a),
$P{F_3}$ contains six electrons in its bond pair and there is one lone pair (two electrons) present in phosphorus, so a total of eight electrons are obtained. $P{F_3}$ satisfies the octet rule. We can draw the structure of this compound as below,
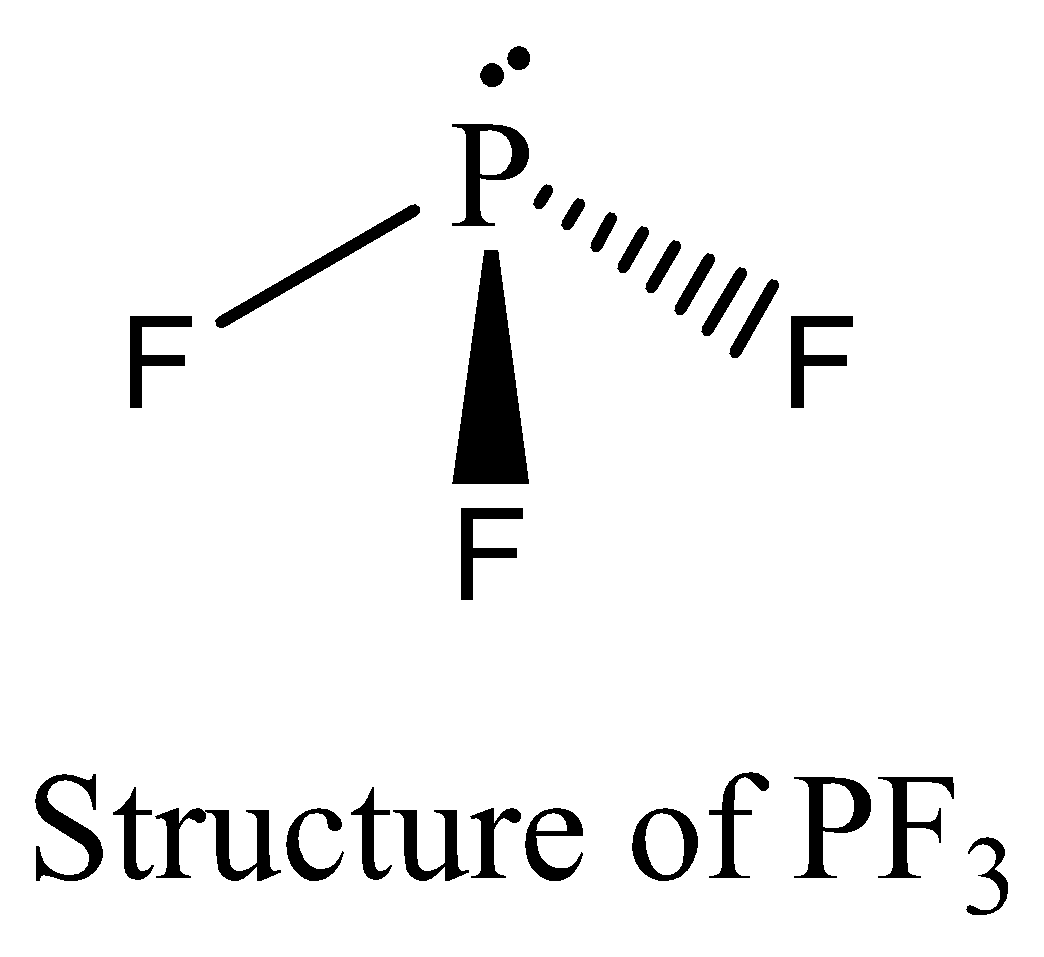
$POC{l_3}$ contains 8 bonding electrons and no lone pair of electrons are present. The octet rule is satisfied by $POC{l_3}$. We can draw the structure of this compound as below,
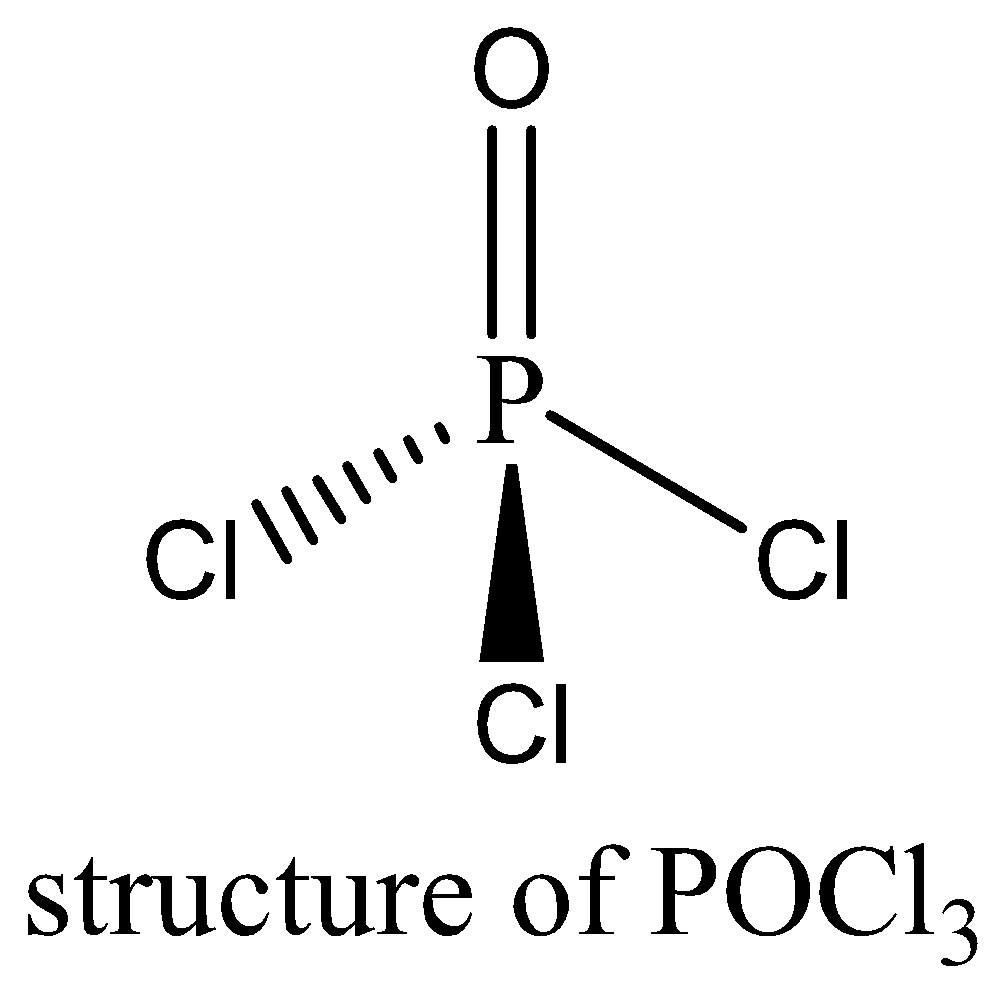
Therefore, the option (A) is incorrect.
In the pair (b),
$BC{l_3}$ contains six electrons in its bond pair and there is no one lone pair present in boron, so a total of eight electrons are not obtained. The octet rule is not satisfied by $BC{l_3}$. We can draw the structure of this compound as below,
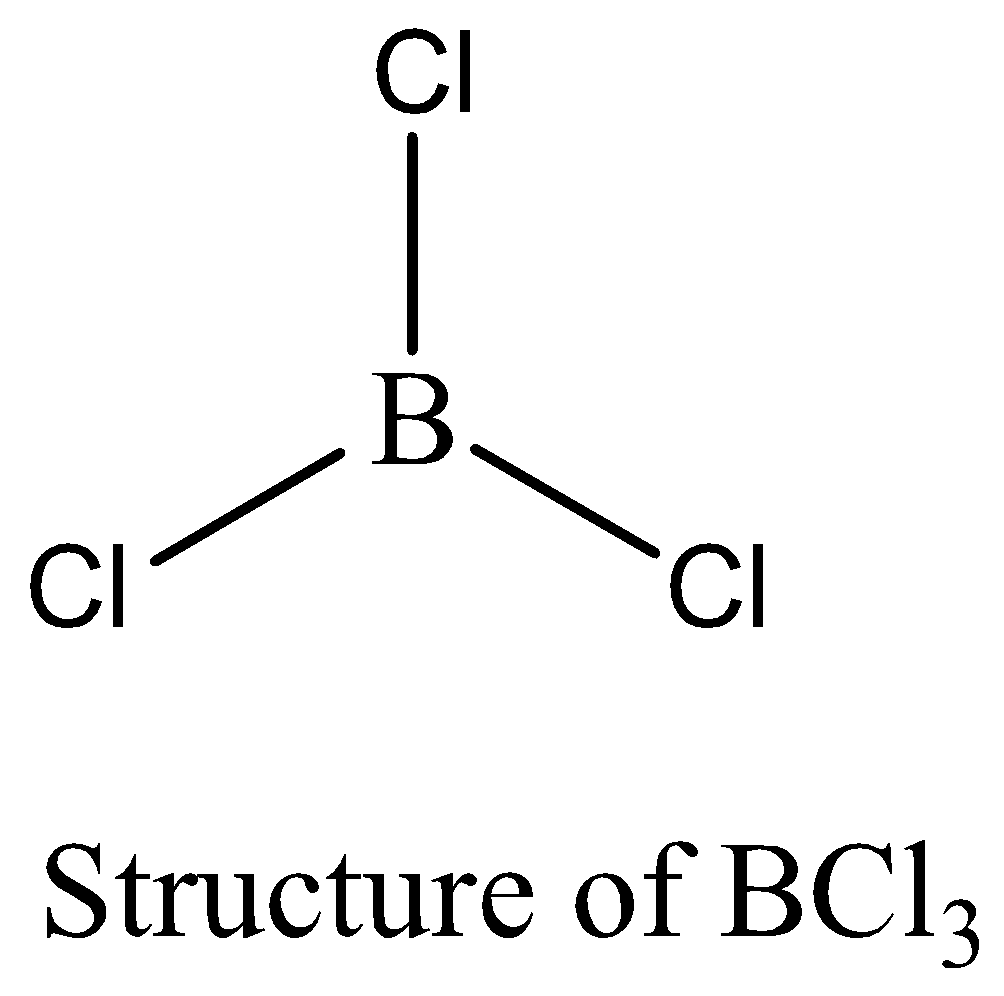
$PC{l_5}$ contains 10 numbers of bonding electrons and no lone pair of electrons are present. The octet rule is not satisfied by $PC{l_5}$. Therefore, the option (B) is correct. We can draw the structure of this compound as below,
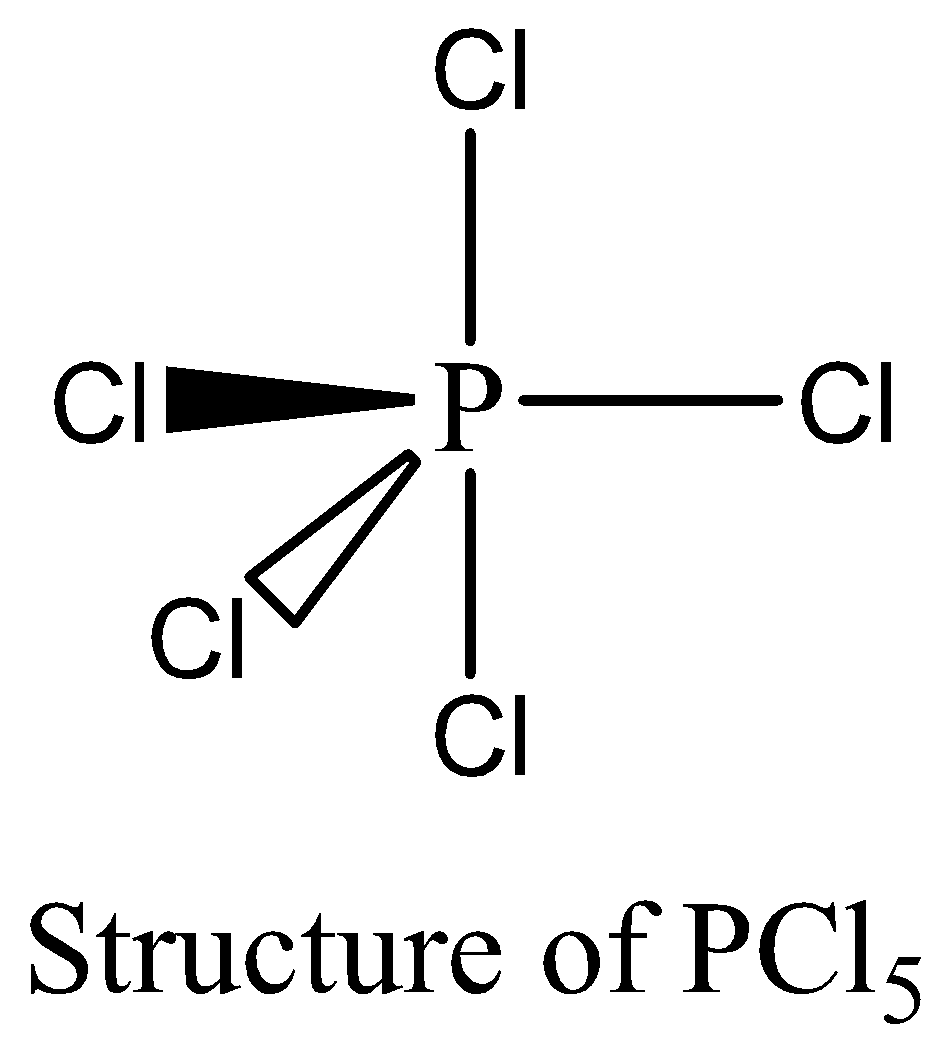
In the pair (c),
$C{F_4}$ contains eight electrons in its bond pair and there is no one lone pair present in carbon, so a total of eight electrons are obtained. The octet rule is satisfied by $C{F_4}$. We can draw the structure of this compound as below,
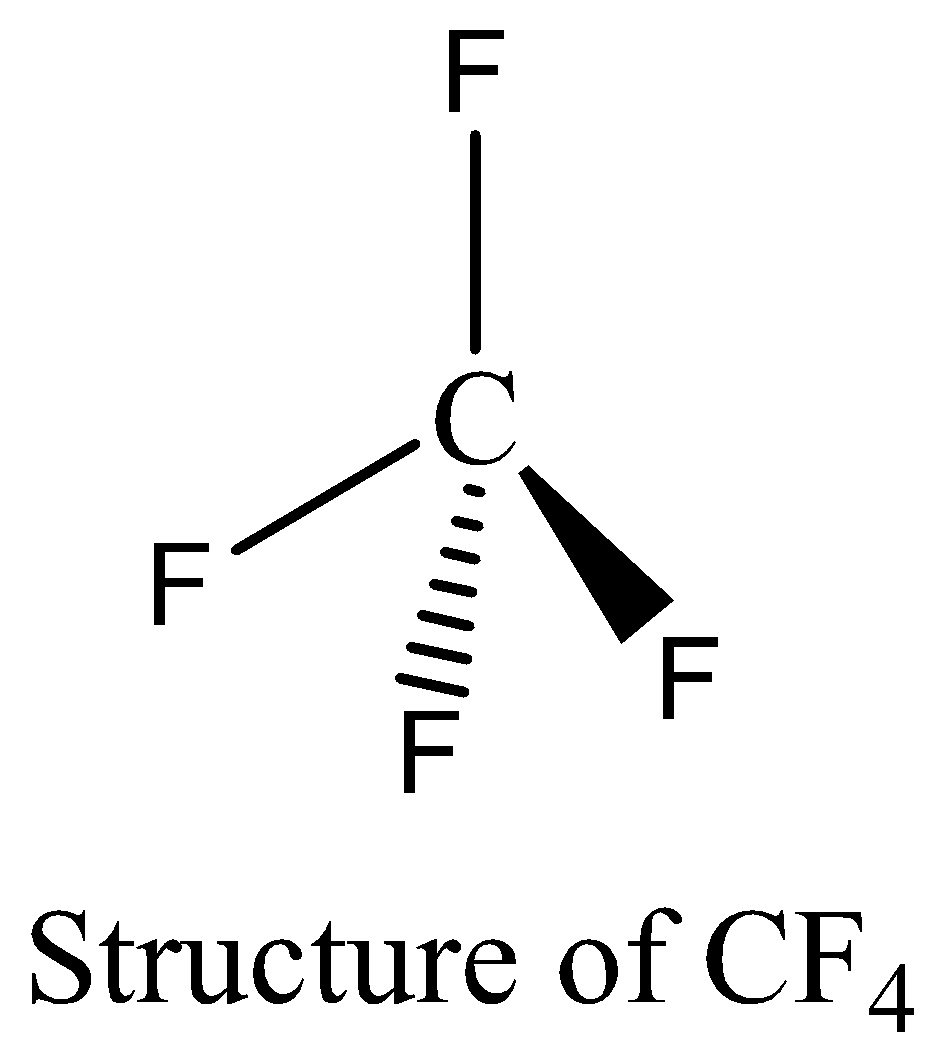
$N{F_3}$ contains six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a total of eight electrons are obtained. The octet rule is satisfied by $N{F_3}$.
We can draw the structure of this compound as below,
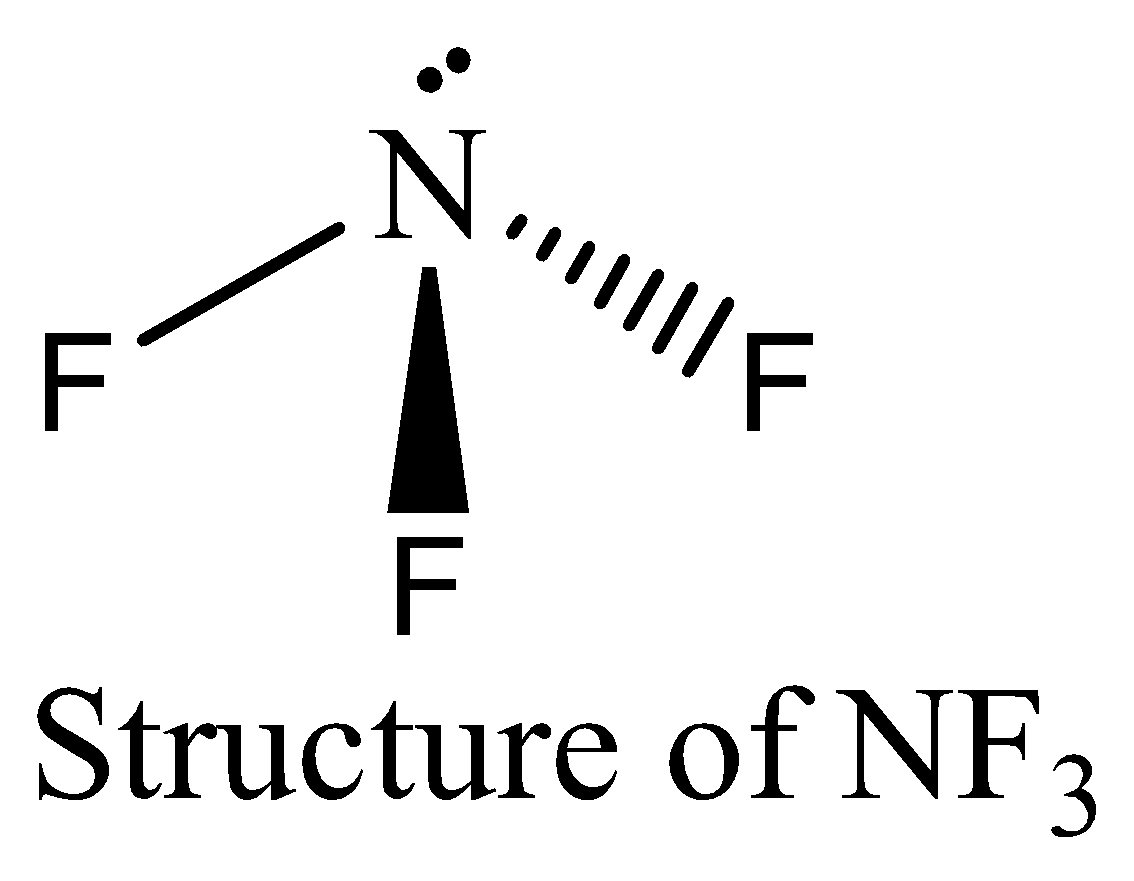
Thus option (C) is incorrect.
In the pair (d),
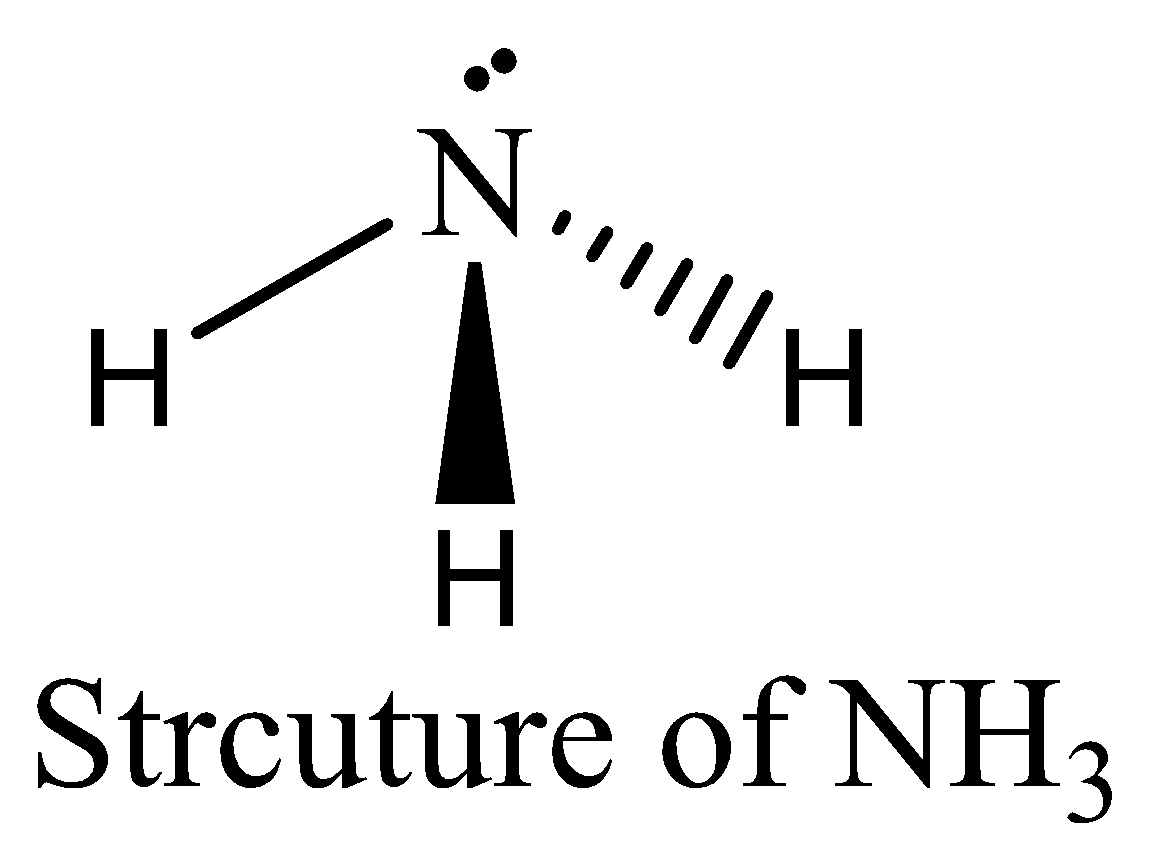
$N{H_3}$ contains six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a total of eight electrons are obtained. The octet rule is satisfied by $N{H_3}$. We can draw the structure of this compound as below,
$NC{l_3}$ contains six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a total of eight electrons are obtained. The octet rule is satisfied by $NC{l_3}$. We can draw the structure of this compound as below,
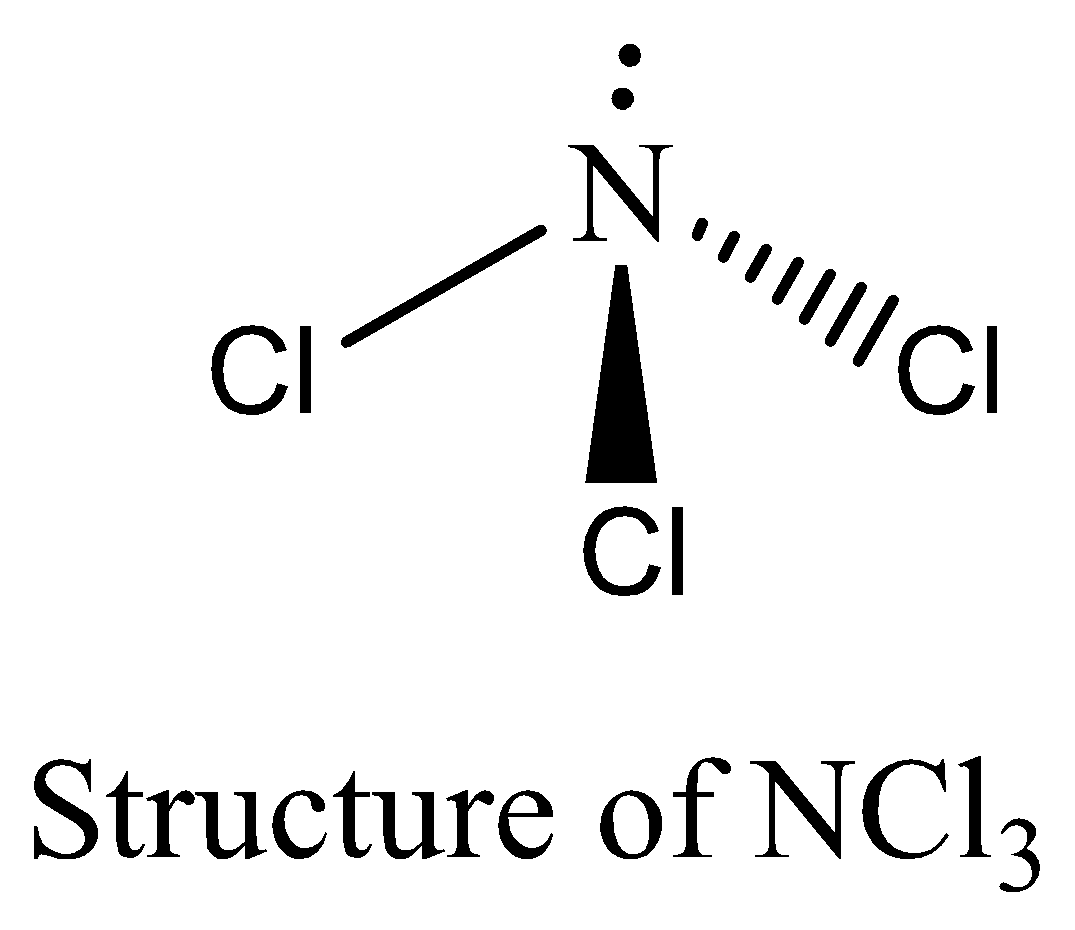
Therefore, the option (D) is incorrect.
The pair of species, which does not follow the octet rule is $BC{l_3}$ and $PC{l_5}$.
Therefore, the correct option is B. .
Note: We must remember that in $S{F_6}$ the atom that violates octet rule is sulfur. The central sulfur atom forms six covalent bonds to six fluorine atoms, therefore it is an expanded valence shell molecule. The atom of sulfur expands its octet, hence the molecule $S{F_6}$ violates the octet rule. In $B{H_3}$ the atom that deviates octet rule is boron. There are only six outermost electrons in $B{H_3}$ around the central atom boron. The atom of boron has incomplete octet and hence, the molecule $B{H_3}$ violates the octet rule.
Complete step by step answer:
We can say a stable arrangement is attained when the atom is surrounded by eight electrons. This octet could be made up of its own electrons and some electrons that are shared. Therefore, an atom continues to form bonds until an octet of electrons is made
Let us now calculate the total number of electrons present in all compounds by adding the electrons in bond pairs and lone pairs.
In the pair (a),
$P{F_3}$ contains six electrons in its bond pair and there is one lone pair (two electrons) present in phosphorus, so a total of eight electrons are obtained. $P{F_3}$ satisfies the octet rule. We can draw the structure of this compound as below,

$POC{l_3}$ contains 8 bonding electrons and no lone pair of electrons are present. The octet rule is satisfied by $POC{l_3}$. We can draw the structure of this compound as below,

Therefore, the option (A) is incorrect.
In the pair (b),
$BC{l_3}$ contains six electrons in its bond pair and there is no one lone pair present in boron, so a total of eight electrons are not obtained. The octet rule is not satisfied by $BC{l_3}$. We can draw the structure of this compound as below,

$PC{l_5}$ contains 10 numbers of bonding electrons and no lone pair of electrons are present. The octet rule is not satisfied by $PC{l_5}$. Therefore, the option (B) is correct. We can draw the structure of this compound as below,

In the pair (c),
$C{F_4}$ contains eight electrons in its bond pair and there is no one lone pair present in carbon, so a total of eight electrons are obtained. The octet rule is satisfied by $C{F_4}$. We can draw the structure of this compound as below,

$N{F_3}$ contains six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a total of eight electrons are obtained. The octet rule is satisfied by $N{F_3}$.
We can draw the structure of this compound as below,

Thus option (C) is incorrect.
In the pair (d),
$N{H_3}$ contains six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a total of eight electrons are obtained. The octet rule is satisfied by $N{H_3}$. We can draw the structure of this compound as below,

$NC{l_3}$ contains six electrons in its bond pair and there is one lone pair (two electrons) present in nitrogen, so a total of eight electrons are obtained. The octet rule is satisfied by $NC{l_3}$. We can draw the structure of this compound as below,

Therefore, the option (D) is incorrect.
The pair of species, which does not follow the octet rule is $BC{l_3}$ and $PC{l_5}$.
Therefore, the correct option is B. .
Note: We must remember that in $S{F_6}$ the atom that violates octet rule is sulfur. The central sulfur atom forms six covalent bonds to six fluorine atoms, therefore it is an expanded valence shell molecule. The atom of sulfur expands its octet, hence the molecule $S{F_6}$ violates the octet rule. In $B{H_3}$ the atom that deviates octet rule is boron. There are only six outermost electrons in $B{H_3}$ around the central atom boron. The atom of boron has incomplete octet and hence, the molecule $B{H_3}$ violates the octet rule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




