
In which pair of ions both the species contains an S-S bond?
A. \[{S_4}O_6^{2 - },{S_2}O_7^{2 - }\]
B. \[{S_2}O_7^{2 - },{S_2}O_3^{2 - }\]
C. \[{S_4}O_6^{2 - },{S_2}O_3^{2 - }\]
D. \[{S_2}O_7^{2 - },{S_2}O_8^{2 - }\]
Answer
577.5k+ views
Hint: An oxide is a binary compound that we obtain upon the reaction of oxygen with other elements. In a molecule the sulphur is bonded to sulphur need to be determined for that the structure of oxoacids need to be known.
Complete answer
Oxides are of three types; one is acidic if oxygen binds with non-metal. Second is basic, if it forms with metal and third is amphoteric, if it bonds with semimetals.
We know that the oxo acid of sulphur is sulphuric acid. Sulphuric acid is highly corrosive and is not easy to handle, special precaution is needed to do experiments which include use of acid as it burns the skin. The structure of sulphuric acid is shown below.

Here, there is no S-S bond. The chemical formula is \[{H_2}S{O_4}\], the structural is tetrahedral. The oxidation state of sulphur atoms is \[ + 6\] and act as a good oxidizing agent.
The structure of sulphurous acid is shown below.
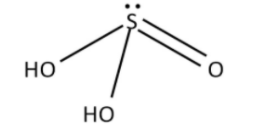
Here, there is no S-S bond. The molecular formula is \[{H_2}S{O_3}\]. The structure is trigonal pyramidal structure. Oxidation state of sulphur is \[ + 4\]. It is a good reducing agent.
The other includes:
\[
{H_2}{S_2}{O_3}(thiosulphuric\;acid)\\
{H_2}{S_2}{O_4}(dithionous\;acid)\\
{H_2}{S_2}{O_5}(sulphurous\;acid)\\
{H_2}{S_x}{O_6}(x = 2\;to\;5)\\
{H_2}{S_2}{O_7}(pyrosulphuric\;acid\;or\;Oleum)\\
{H_2}S{O_5}(pyrooxymomosulphuric\;acid)\\
{H_2}{S_2}{O_8}(peroxodisulphurous\;acid)
\]
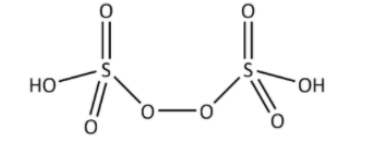
\[{H_2}{S_2}{O_7}(pyrosulphuric\;acid\;or\;Oleum)\]have the following structure,

\[{H_2}{S_2}{O_8}(peroxodisulphurous\;acid)\] have the following structure,
The structures of \[{S_4}O_6^{2 - }\;and\;{S_2}O_3^{2 - }\] are shown below.

Therefore, from the above structures, we conclude that the pair of ions in which both the species contains S-S bond is \[{S_4}O_6^{2 - },{S_2}O_3^{2 - }\].
Hence the correct answer is (C).
Note:
There should be more than one sulphur atom should be present in the molecule. Then only the double bond between the sulphur atom is possible in the oxides of sulphur.
Complete answer
Oxides are of three types; one is acidic if oxygen binds with non-metal. Second is basic, if it forms with metal and third is amphoteric, if it bonds with semimetals.
We know that the oxo acid of sulphur is sulphuric acid. Sulphuric acid is highly corrosive and is not easy to handle, special precaution is needed to do experiments which include use of acid as it burns the skin. The structure of sulphuric acid is shown below.

Here, there is no S-S bond. The chemical formula is \[{H_2}S{O_4}\], the structural is tetrahedral. The oxidation state of sulphur atoms is \[ + 6\] and act as a good oxidizing agent.
The structure of sulphurous acid is shown below.

Here, there is no S-S bond. The molecular formula is \[{H_2}S{O_3}\]. The structure is trigonal pyramidal structure. Oxidation state of sulphur is \[ + 4\]. It is a good reducing agent.
The other includes:
\[
{H_2}{S_2}{O_3}(thiosulphuric\;acid)\\
{H_2}{S_2}{O_4}(dithionous\;acid)\\
{H_2}{S_2}{O_5}(sulphurous\;acid)\\
{H_2}{S_x}{O_6}(x = 2\;to\;5)\\
{H_2}{S_2}{O_7}(pyrosulphuric\;acid\;or\;Oleum)\\
{H_2}S{O_5}(pyrooxymomosulphuric\;acid)\\
{H_2}{S_2}{O_8}(peroxodisulphurous\;acid)
\]
\[{H_2}{S_2}{O_7}(pyrosulphuric\;acid\;or\;Oleum)\]have the following structure,

\[{H_2}{S_2}{O_8}(peroxodisulphurous\;acid)\] have the following structure,

The structures of \[{S_4}O_6^{2 - }\;and\;{S_2}O_3^{2 - }\] are shown below.

Therefore, from the above structures, we conclude that the pair of ions in which both the species contains S-S bond is \[{S_4}O_6^{2 - },{S_2}O_3^{2 - }\].
Hence the correct answer is (C).
Note:
There should be more than one sulphur atom should be present in the molecule. Then only the double bond between the sulphur atom is possible in the oxides of sulphur.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




